当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The structural basis of N-acyl-α-amino-β-lactone formation catalyzed by a nonribosomal peptide synthetase.
Nature Communications ( IF 14.7 ) Pub Date : 2019-07-31 , DOI: 10.1038/s41467-019-11383-7 Dale F Kreitler 1 , Erin M Gemmell 2 , Jason E Schaffer 2 , Timothy A Wencewicz 2 , Andrew M Gulick 1
Nature Communications ( IF 14.7 ) Pub Date : 2019-07-31 , DOI: 10.1038/s41467-019-11383-7 Dale F Kreitler 1 , Erin M Gemmell 2 , Jason E Schaffer 2 , Timothy A Wencewicz 2 , Andrew M Gulick 1
Affiliation
|
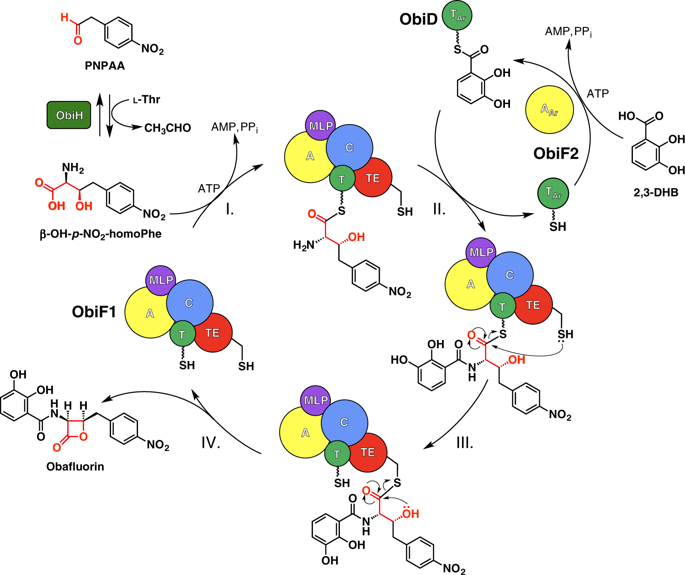
Nonribosomal peptide synthetases produce diverse natural products using a multidomain architecture where the growing peptide, attached to an integrated carrier domain, is delivered to neighboring catalytic domains for bond formation and modification. Investigation of these systems can lead to the discovery of new structures, unusual biosynthetic transformations, and to the engineering of catalysts for generating new products. The antimicrobial β-lactone obafluorin is produced nonribosomally from dihydroxybenzoic acid and a β-hydroxy amino acid that cyclizes into the β-lactone during product release. Here we report the structure of the nonribosomal peptide synthetase ObiF1, highlighting the structure of the β-lactone-producing thioesterase domain and an interaction between the C-terminal MbtH-like domain with an upstream adenylation domain. Biochemical assays examine catalytic promiscuity, provide mechanistic insight, and demonstrate utility for generating obafluorin analogs. These results advance our understanding of the structural cycle of nonribosomal peptide synthetases and provide insights into the production of β-lactone natural products.
中文翻译:

非核糖体肽合成酶催化N-酰基-α-氨基-β-内酯形成的结构基础。
非核糖体肽合成酶使用多结构域结构产生多种天然产物,其中与整合的载体结构域连接的生长中的肽被递送至相邻的催化结构域以形成键和修饰。对这些系统的研究可能会导致发现新结构,异常的生物合成转化,并导致产生新产品的催化剂工程化。抗菌β-内酯氧氟醚非核糖体是由二羟基苯甲酸和在产品释放过程中环化成β-内酯的β-羟基氨基酸非核糖体产生的。在这里,我们报告了非核糖体肽合成酶ObiF1的结构,突出了产生β-内酯的硫酯酶结构域的结构以及C端MbtH样结构域与上游腺苷酸化结构域之间的相互作用。生化分析检查了催化的混杂性,提供了机理上的见解,并证明了产生氧氟烷类似物的实用性。这些结果提高了我们对非核糖体肽合成酶的结构周期的理解,并为β-内酯天然产物的生产提供了见识。
更新日期:2019-07-31
中文翻译:

非核糖体肽合成酶催化N-酰基-α-氨基-β-内酯形成的结构基础。
非核糖体肽合成酶使用多结构域结构产生多种天然产物,其中与整合的载体结构域连接的生长中的肽被递送至相邻的催化结构域以形成键和修饰。对这些系统的研究可能会导致发现新结构,异常的生物合成转化,并导致产生新产品的催化剂工程化。抗菌β-内酯氧氟醚非核糖体是由二羟基苯甲酸和在产品释放过程中环化成β-内酯的β-羟基氨基酸非核糖体产生的。在这里,我们报告了非核糖体肽合成酶ObiF1的结构,突出了产生β-内酯的硫酯酶结构域的结构以及C端MbtH样结构域与上游腺苷酸化结构域之间的相互作用。生化分析检查了催化的混杂性,提供了机理上的见解,并证明了产生氧氟烷类似物的实用性。这些结果提高了我们对非核糖体肽合成酶的结构周期的理解,并为β-内酯天然产物的生产提供了见识。































 京公网安备 11010802027423号
京公网安备 11010802027423号