当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Crystal Structures and Energy Storage Properties of Ammine Sodium Decahydro-closo-decaboranes (Na2B10H10·nNH3, n = 1, 2)
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-08-13 , DOI: 10.1021/acs.jpcc.9b06084 Mathias Jørgensen 1 , Bjarne R. S. Hansen 2 , Young-Su Lee 3 , Young Whan Cho 3 , Torben R. Jensen 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-08-13 , DOI: 10.1021/acs.jpcc.9b06084 Mathias Jørgensen 1 , Bjarne R. S. Hansen 2 , Young-Su Lee 3 , Young Whan Cho 3 , Torben R. Jensen 1
Affiliation
|
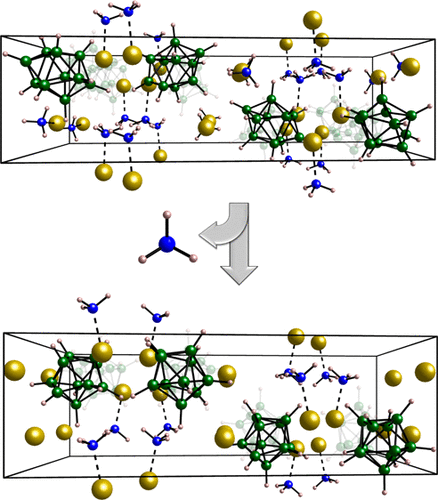
Metal closo-boranes show remarkable thermal and chemical stabilities, making them appealing candidates for a wide range of applications, such as electrolytes in electrochemical batteries and ammonia storage for indirect hydrogen storage. Furthermore, owing to the large size and nonspherical geometry of the anion (e.g., B10H102– or B12H122–), metal closo-boranes display a rich structural diversity and thermal polymorphism. Here, we present the synthesis, characterization, and structural determination of the ammoniated metal closo-boranes, Na2B10H10·nNH3 (n = 1, 2). The thermal decomposition of Na2B10H10·2NH3 was investigated with synchrotron radiation in situ powder X-ray diffraction and simultaneous thermogravimetric analysis, differential scanning calorimetry, and mass spectrometry, revealing a reversible ammonia storage capacity of 15 wt % below 150 °C. Additionally, ionic conductivities of 2.7 × 10–8 (RT) and 4.7 × 10–8 S/cm (30 °C) for Na2B10H10·2NH3 and Na2B10H10·NH3, respectively, were measured with electrochemical impedance spectroscopy. A lower Na+ conductivity compared to the parent compound, Na2B10H10, is explained by an anchoring effect of ammonia in the rigid framework of the B10H102–-anions.
中文翻译:

氨基十氢闭环十硼烷(Na2B10H10·nNH3,n = 1,2)的晶体结构和储能性能
金属闭合碳-boranes显示显着的热稳定性和化学稳定性,使它们呼吁提供广泛的应用,例如在电化学电池和氨储存用于间接储氢电解质候选。此外,由于阴离子的大尺寸和非球形几何形状(例如,B 10 H ^ 10 2-或B 12 ħ 12 2- ),金属闭合碳-boranes显示丰富的结构多样性和热多态性。这里,我们提出的合成,表征,和氨化金属的结构测定闭合碳-boranes,钠2乙10 ħ 10 ·n NH 3(n = 1,2)。Na 2 B 10 H 10 ·2NH 3的热分解通过同步辐射原位粉末X射线衍射,同时热重分析,差示扫描量热法和质谱法进行研究,发现在150以下时可逆的氨存储能力为15 wt%。 ℃。此外,Na 2 B 10 H 10 ·2NH 3和Na 2 B 10 H 10 ·NH的离子电导率分别为2.7×10 –8(RT)和4.7×10 –8 S / cm(30°C)用电化学阻抗光谱法分别测量了图3中的样品。与母体化合物Na 2 B 10 H 10相比,Na +的电导率较低,这是由于氨在B 10 H 10 2-阴离子的刚性骨架中的锚固作用所致。
更新日期:2019-08-14
中文翻译:

氨基十氢闭环十硼烷(Na2B10H10·nNH3,n = 1,2)的晶体结构和储能性能
金属闭合碳-boranes显示显着的热稳定性和化学稳定性,使它们呼吁提供广泛的应用,例如在电化学电池和氨储存用于间接储氢电解质候选。此外,由于阴离子的大尺寸和非球形几何形状(例如,B 10 H ^ 10 2-或B 12 ħ 12 2- ),金属闭合碳-boranes显示丰富的结构多样性和热多态性。这里,我们提出的合成,表征,和氨化金属的结构测定闭合碳-boranes,钠2乙10 ħ 10 ·n NH 3(n = 1,2)。Na 2 B 10 H 10 ·2NH 3的热分解通过同步辐射原位粉末X射线衍射,同时热重分析,差示扫描量热法和质谱法进行研究,发现在150以下时可逆的氨存储能力为15 wt%。 ℃。此外,Na 2 B 10 H 10 ·2NH 3和Na 2 B 10 H 10 ·NH的离子电导率分别为2.7×10 –8(RT)和4.7×10 –8 S / cm(30°C)用电化学阻抗光谱法分别测量了图3中的样品。与母体化合物Na 2 B 10 H 10相比,Na +的电导率较低,这是由于氨在B 10 H 10 2-阴离子的刚性骨架中的锚固作用所致。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号