当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Third Generation of Artificial Dye-Decolorizing Peroxidase Rationally Designed in Myoglobin
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-07-29 00:00:00 , DOI: 10.1021/acscatal.9b02226 Ping Zhang 1 , Jiakun Xu 2 , Xiao-Juan Wang 1 , Bo He 1 , Shu-Qin Gao 3 , Ying-Wu Lin 1, 3
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-07-29 00:00:00 , DOI: 10.1021/acscatal.9b02226 Ping Zhang 1 , Jiakun Xu 2 , Xiao-Juan Wang 1 , Bo He 1 , Shu-Qin Gao 3 , Ying-Wu Lin 1, 3
Affiliation
|
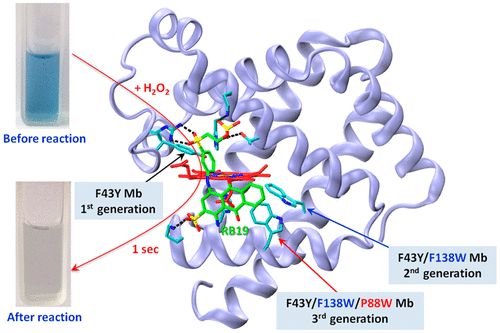
Approaches to degradation of industrial dyes are desirable, of which bioremediation is more favorable. In addition to the use of native enzymes, rational design of artificial enzymes provides an alternative approach. Meanwhile, few designs can achieve a catalytic activity comparable to that of native enzymes. We have previously designed two generations of artificial dye-decolorizing peroxidases (DyPs) in myoglobin (Mb) by introduction of Tyr43 and Trp138 in the heme pocket; however, the activity is moderate. To improve the activity of the artificial DyP, we herein designed a third generation by introduction of an additional Trp (P88W) to the protein surface, named F43Y/F138W/P88W Mb. The third generation of artificial DyP was shown to exhibit a catalytic efficiency exceeding that of various native DyPs and comparable to that of the most efficient native DyPs. Titration of reactive blue 19 (RB19) and molecular docking studies revealed crucial roles of Trp88 in substrate binding and oxidation, which acts as a catalytic site. This study not only provides clues for heme protein design but also suggests that the artificial DyP has potential applications for bioremediation in the future.
中文翻译:

在肌红蛋白中合理设计的第三代人工染料脱色过氧化物酶
期望降解工业染料的方法,其中生物修复是更有利的。除了使用天然酶外,合理设计人造酶还提供了另一种方法。同时,很少有设计能够达到与天然酶相当的催化活性。我们以前通过在血红素袋中引入Tyr43和Trp138,设计了两代肌红蛋白(Mb)中的人造染料脱色过氧化物酶(DyPs);但是,活动是中等的。为了提高人工DyP的活性,我们在本文中设计了第三代,方法是将另外的Trp(P88W)引入蛋白表面,称为F43Y / F138W / P88W Mb。已显示第三代人工DyP显示出超过各种天然DyP的催化效率,并且可与最有效的天然DyP相媲美。活性蓝19(RB19)的滴定和分子对接研究表明,Trp88在底物结合和氧化中起着至关重要的作用,后者是一个催化位点。这项研究不仅为血红素蛋白设计提供了线索,而且表明人工DyP在未来的生物修复中具有潜在的应用。
更新日期:2019-07-29
中文翻译:

在肌红蛋白中合理设计的第三代人工染料脱色过氧化物酶
期望降解工业染料的方法,其中生物修复是更有利的。除了使用天然酶外,合理设计人造酶还提供了另一种方法。同时,很少有设计能够达到与天然酶相当的催化活性。我们以前通过在血红素袋中引入Tyr43和Trp138,设计了两代肌红蛋白(Mb)中的人造染料脱色过氧化物酶(DyPs);但是,活动是中等的。为了提高人工DyP的活性,我们在本文中设计了第三代,方法是将另外的Trp(P88W)引入蛋白表面,称为F43Y / F138W / P88W Mb。已显示第三代人工DyP显示出超过各种天然DyP的催化效率,并且可与最有效的天然DyP相媲美。活性蓝19(RB19)的滴定和分子对接研究表明,Trp88在底物结合和氧化中起着至关重要的作用,后者是一个催化位点。这项研究不仅为血红素蛋白设计提供了线索,而且表明人工DyP在未来的生物修复中具有潜在的应用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号