当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Better Choice of Tertiary Alkanolamines for Postcombustion CO2 Capture: Structure with Linear Alkanol Chain Instead of Branched
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2019-08-09 , DOI: 10.1021/acs.iecr.9b02244
Sen Liu 1 , Hao Ling 1 , Hongxia Gao 1 , Paitoon Tontiwachwuthikul 1 , Zhiwu Liang 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2019-08-09 , DOI: 10.1021/acs.iecr.9b02244
Sen Liu 1 , Hao Ling 1 , Hongxia Gao 1 , Paitoon Tontiwachwuthikul 1 , Zhiwu Liang 1
Affiliation
|
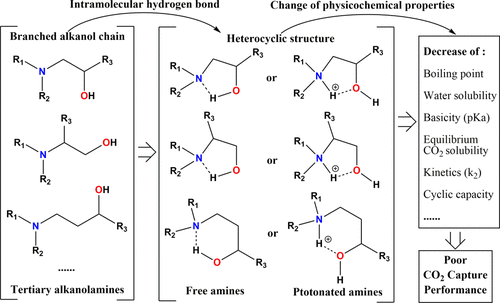
To give better guidance of tertiary alkanolamine selection with proper structures for postcombustion CO2 capture, effects of the alkanol chain structure and intramolecular hydrogen bond were investigated. First, water solubilities of 2.5 M aqueous solutions were experimentally investigated for 11 tertiary alkanolamines, and data of boiling point and vapor pressure for pure chemicals were collected also. The physical property results showed a biphasic phenomenon were observed in 2.5 M fresh 1-diethylamino-2-propanol (1DEA2P) solution and CO2-loaded 4-diethylamino-2-butanol (4DEA-2B) solution, and 1-dimethylamino-2-propanol (1DMA2P) and 1DEA2P have a dramatically relative lower boiling point and a higher vapor pressure, indicating the existence of an intramolecular hydrogen bond, especially in amines with branched alkanol chains. In addition, the pH values for fresh and equilibrium CO2-loaded amine solutions (2.5 M, 313 K), equilibrium CO2 solubility (2.5 M, 313 K, and 15 kPa), first-order reaction rate constant (0.1–0.4 M and 313 K), and dissociation constant (293–333 K) were measured, and the results showed amines with linear alkanol chains can have relatively strong basicity, absorption capacity, and a reaction rate with CO2 owing to weaker influences of the intramolecular hydrogen bond. Finally, the CO2 capture performance was comprehensively compared by ΔrGm and ΔrHm screening methods as well as the fast solvent screening method with cyclic capacity, and the results further confirmed that amines with linear alkanol chains can present better CO2 capture performance, like 3-dimethylamino-1-propanol (3DMA1P) and 3-diethylamino-1-propanol (3DEA1P). It also suggests that the selection or design of tertiary alkanolamines should with the linear alkanol chain instead of branched to have better CO2 capture performance for industrial applications.
中文翻译:

用于燃烧后二氧化碳捕集的叔烷醇胺的更好选择:具有线性烷醇链而不是支链的结构
为了更好地指导叔链烷醇胺的选择,并采用适当的结构进行燃烧后CO 2捕获,研究了链烷醇链结构和分子内氢键的影响。首先,通过实验研究了2.5 M水溶液对11种叔链烷醇胺的水溶性,并且还收集了纯化学品的沸点和蒸气压数据。物理性质结果表明,在2.5 M新鲜的1-二乙基氨基-2-丙醇(1DEA2P)溶液和CO 2中观察到两相现象。负载的4-二乙基氨基-2-丁醇(4DEA-2B)溶液和1-二甲基氨基-2-丙醇(1DMA2P)和1DEA2P具有相对较低的沸点和较高的蒸气压,表明存在分子内氢键,尤其是在具有支链烷醇链的胺中。此外,新鲜和平衡的装载有CO 2的胺溶液的pH值(2.5 M,313 K),平衡的CO 2溶解度(2.5 M,313 K和15 kPa),一级反应速率常数(0.1-0.4) M和313 K),以及解离常数(293-333 K),结果表明,具有直链烷醇链的胺可以具有较强的碱度,吸收能力和与CO 2的反应速率由于分子内氢键的影响较弱。最后,通过Δr G m和Δr H m筛选方法以及具有循环容量的快速溶剂筛选方法,对CO 2捕获性能进行了全面比较,结果进一步证实具有直链烷醇链的胺可以表现出更好的CO 2。捕获性能,如3-二甲基氨基-1-丙醇(3DMA1P)和3-二乙基氨基-1-丙醇(3DEA1P)。这也表明叔链烷醇胺的选择或设计应带有直链烷醇链而不是支链,以具有更好的工业应用CO 2捕集性能。
更新日期:2019-08-10
中文翻译:

用于燃烧后二氧化碳捕集的叔烷醇胺的更好选择:具有线性烷醇链而不是支链的结构
为了更好地指导叔链烷醇胺的选择,并采用适当的结构进行燃烧后CO 2捕获,研究了链烷醇链结构和分子内氢键的影响。首先,通过实验研究了2.5 M水溶液对11种叔链烷醇胺的水溶性,并且还收集了纯化学品的沸点和蒸气压数据。物理性质结果表明,在2.5 M新鲜的1-二乙基氨基-2-丙醇(1DEA2P)溶液和CO 2中观察到两相现象。负载的4-二乙基氨基-2-丁醇(4DEA-2B)溶液和1-二甲基氨基-2-丙醇(1DMA2P)和1DEA2P具有相对较低的沸点和较高的蒸气压,表明存在分子内氢键,尤其是在具有支链烷醇链的胺中。此外,新鲜和平衡的装载有CO 2的胺溶液的pH值(2.5 M,313 K),平衡的CO 2溶解度(2.5 M,313 K和15 kPa),一级反应速率常数(0.1-0.4) M和313 K),以及解离常数(293-333 K),结果表明,具有直链烷醇链的胺可以具有较强的碱度,吸收能力和与CO 2的反应速率由于分子内氢键的影响较弱。最后,通过Δr G m和Δr H m筛选方法以及具有循环容量的快速溶剂筛选方法,对CO 2捕获性能进行了全面比较,结果进一步证实具有直链烷醇链的胺可以表现出更好的CO 2。捕获性能,如3-二甲基氨基-1-丙醇(3DMA1P)和3-二乙基氨基-1-丙醇(3DEA1P)。这也表明叔链烷醇胺的选择或设计应带有直链烷醇链而不是支链,以具有更好的工业应用CO 2捕集性能。

































 京公网安备 11010802027423号
京公网安备 11010802027423号