当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gold(i)-catalyzed stereoselective cyclization of 1,3-enyne aldehydes by a 1,3-acyloxy migration/Nazarov cyclization/aldol addition cascade.
Chemical Science ( IF 7.6 ) Pub Date : 2019-07-29 , DOI: 10.1039/c9sc02828e Marco Brandstätter 1 , Nikolas Huwyler 1 , Erick M Carreira 1
Chemical Science ( IF 7.6 ) Pub Date : 2019-07-29 , DOI: 10.1039/c9sc02828e Marco Brandstätter 1 , Nikolas Huwyler 1 , Erick M Carreira 1
Affiliation
|
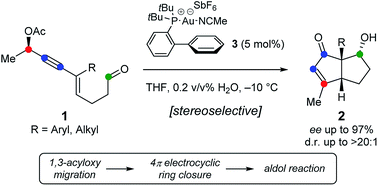
Stereoselective synthesis of bicyclo[3.3.0]octenones from chiral 1,3-enyne aldehydes bearing propargylic acetates is described. The method is based on a Au(i)-catalyzed domino sequence with concomitant transfer of chirality involving 1,3-acyloxy migration followed by Nazarov cyclization and an unprecedented aldol addition. The method furnishes densely functionalized bicyclic structures in high yields, with up to 97% ee and good diastereoselectivity.
中文翻译:

通过1,3-酰氧基迁移/纳扎罗夫环化/醛醇加成级联反应的金(i)催化的1,3-烯炔醛的立体选择性环化。
描述了由带有炔丙基乙酸酯的手性1,3-烯炔醛立体选择性地合成双环[3.3.0]辛烯酮。该方法基于Au(i)催化的多米诺骨牌序列,伴随手性转移,涉及1,3-酰氧基迁移,然后进行Nazarov环化和前所未有的醛醇加成。该方法可提供高收率的致密功能化双环结构,ee最高可达97%,非对映选择性良好。
更新日期:2019-07-29
中文翻译:

通过1,3-酰氧基迁移/纳扎罗夫环化/醛醇加成级联反应的金(i)催化的1,3-烯炔醛的立体选择性环化。
描述了由带有炔丙基乙酸酯的手性1,3-烯炔醛立体选择性地合成双环[3.3.0]辛烯酮。该方法基于Au(i)催化的多米诺骨牌序列,伴随手性转移,涉及1,3-酰氧基迁移,然后进行Nazarov环化和前所未有的醛醇加成。该方法可提供高收率的致密功能化双环结构,ee最高可达97%,非对映选择性良好。






























 京公网安备 11010802027423号
京公网安备 11010802027423号