当前位置:
X-MOL 学术
›
STEM CELLS
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
SMYD2 Drives Mesendodermal Differentiation of Human Embryonic Stem Cells through Mediating the Transcriptional Activation of Key Mesendodermal Genes
STEM CELLS ( IF 4.0 ) Pub Date : 2019-08-12 , DOI: 10.1002/stem.3068
Hua-Jun Bai 1 , Peng Zhang 1 , Li Ma 2 , He Liang 1 , Gang Wei 2 , Huang-Tian Yang 1
STEM CELLS ( IF 4.0 ) Pub Date : 2019-08-12 , DOI: 10.1002/stem.3068
Hua-Jun Bai 1 , Peng Zhang 1 , Li Ma 2 , He Liang 1 , Gang Wei 2 , Huang-Tian Yang 1
Affiliation
|
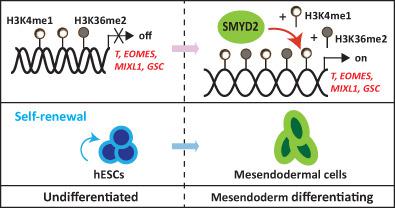
Histone methyltransferases play a critical role in early human development, whereas their roles and precise mechanisms are less understood. SET and MYND domain‐containing protein 2 (SMYD2) is a histone lysine methyltransferase induced during early differentiation of human embryonic stem cells (hESCs), but little is known about its function in undifferentiated hESCs and in their early lineage fate decision as well as underlying mechanisms. Here, we explored the role of SMYD2 in the self‐renewal and mesendodermal lineage commitment of hESCs. We demonstrated that the expression of SMYD2 was significantly enhanced during mesendodermal but not neuroectodermal differentiation of hESCs. SMYD2 knockout (SMYD2−/−) did not affect self‐renewal and early neuroectodermal differentiation of hESCs, whereas it blocked the mesendodermal lineage commitment. This phenotype was rescued by reintroduction of SMYD2 into the SMYD2−/− hESCs. Mechanistically, the bindings of SMYD2 at the promoter regions of critical mesendodermal transcription factor genes, namely, brachyury (T), eomesodermin (EOMES), mix paired‐like homeobox (MIXL1), and goosecoid homeobox (GSC) were significantly enhanced during mesendodermal differentiation of SMYD2+/+ hESCs but totally suppressed in SMYD2−/− ones. Concomitantly, such a suppression was associated with the remarkable reduction of methylation at histone 3 lysine 4 and lysine 36 but not at histone 4 lysine 20 globally and specifically on the promoter regions of mesendodermal genes, namely, T, EOMES, MIXL1, and GSC. These results reveal that the histone methyltransferase SMYD2 is dispensable in the undifferentiated hESCs and the early neuroectodermal differentiation, but it promotes the mesendodermal differentiation of hESCs through the epigenetic control of critical genes to mesendodermal lineage commitment. Stem Cells 2019;37:1401–1415
中文翻译:

SMYD2通过介导中内胚层关键基因的转录激活来驱动人胚胎干细胞的中内胚层分化
组蛋白甲基转移酶在早期人类发育中发挥着关键作用,而它们的作用和精确机制却鲜为人知。SET 和 MYND 结构域蛋白 2 (SMYD2) 是人类胚胎干细胞 (hESC) 早期分化过程中诱导的组蛋白赖氨酸甲基转移酶,但对其在未分化 hESC 及其早期谱系命运决定中的功能知之甚少。机制。在这里,我们探讨了 SMYD2 在 hESC 的自我更新和中内胚层谱系承诺中的作用。我们证明 SMYD2 的表达在 hESC 的中内胚层而非神经外胚层分化过程中显着增强。SMYD2 敲除 (SMYD2-/-) 不影响 hESC 的自我更新和早期神经外胚层分化,但它阻止了中内胚层谱系的承诺。这种表型通过将 SMYD2 重新引入 SMYD2-/- hESC 中得以挽救。从机制上讲,SMYD2在关键中内胚层转录因子基因的启动子区域的结合,即brachyury (T)、eomesodermin (EOMES)、混合配对样同源盒(MIXL1)和鹅卵形同源盒(GSC)在中内胚层分化过程中显着增强SMYD2+/+ hESC,但在 SMYD2−/− 中完全受到抑制。同时,这种抑制与组蛋白 3 赖氨酸 4 和赖氨酸 36 甲基化的显着降低有关,但与组蛋白 4 赖氨酸 20 的甲基化无关,尤其是在中内胚层基因的启动子区域,即 T、EOMES、MIXL1 和 GSC。这些结果表明组蛋白甲基转移酶 SMYD2 在未分化的 hESC 和早期神经外胚层分化中是可有可无的,但它通过关键基因对中内胚层谱系的表观遗传控制促进了 hESC 的中内胚层分化。干细胞 2019;37:1401–1415
更新日期:2019-08-12
中文翻译:

SMYD2通过介导中内胚层关键基因的转录激活来驱动人胚胎干细胞的中内胚层分化
组蛋白甲基转移酶在早期人类发育中发挥着关键作用,而它们的作用和精确机制却鲜为人知。SET 和 MYND 结构域蛋白 2 (SMYD2) 是人类胚胎干细胞 (hESC) 早期分化过程中诱导的组蛋白赖氨酸甲基转移酶,但对其在未分化 hESC 及其早期谱系命运决定中的功能知之甚少。机制。在这里,我们探讨了 SMYD2 在 hESC 的自我更新和中内胚层谱系承诺中的作用。我们证明 SMYD2 的表达在 hESC 的中内胚层而非神经外胚层分化过程中显着增强。SMYD2 敲除 (SMYD2-/-) 不影响 hESC 的自我更新和早期神经外胚层分化,但它阻止了中内胚层谱系的承诺。这种表型通过将 SMYD2 重新引入 SMYD2-/- hESC 中得以挽救。从机制上讲,SMYD2在关键中内胚层转录因子基因的启动子区域的结合,即brachyury (T)、eomesodermin (EOMES)、混合配对样同源盒(MIXL1)和鹅卵形同源盒(GSC)在中内胚层分化过程中显着增强SMYD2+/+ hESC,但在 SMYD2−/− 中完全受到抑制。同时,这种抑制与组蛋白 3 赖氨酸 4 和赖氨酸 36 甲基化的显着降低有关,但与组蛋白 4 赖氨酸 20 的甲基化无关,尤其是在中内胚层基因的启动子区域,即 T、EOMES、MIXL1 和 GSC。这些结果表明组蛋白甲基转移酶 SMYD2 在未分化的 hESC 和早期神经外胚层分化中是可有可无的,但它通过关键基因对中内胚层谱系的表观遗传控制促进了 hESC 的中内胚层分化。干细胞 2019;37:1401–1415

































 京公网安备 11010802027423号
京公网安备 11010802027423号