当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Synthesis of Azepine-Fused Cyclobutanes from Yne-Methylenecyclopropanes Involving Cyclopropanation/C–C Cleavage/Wagner–Meerwein Rearrangement and Reaction Mechanism
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-07-26 00:00:00 , DOI: 10.1021/acs.joc.9b01071 Chen-Long Li 1 , Zhi-Xiang Yu 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-07-26 00:00:00 , DOI: 10.1021/acs.joc.9b01071 Chen-Long Li 1 , Zhi-Xiang Yu 1
Affiliation
|
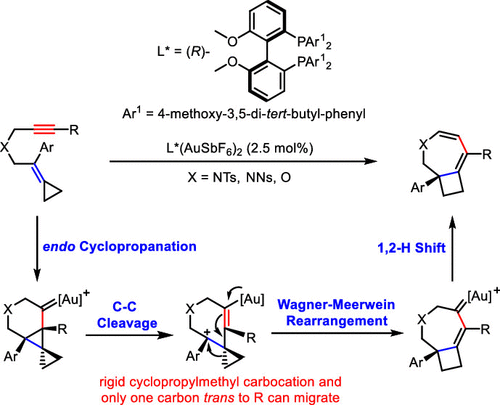
Ring expansion of in situ generated cyclopropylmethyl cations via Wagner–Meerwein rearrangement to cyclobutanes is widely used in synthesis. However, the cyclopropylmethyl cations generated are planar, which would lead to loss of chiral information in the case of chiral precursors, making an asymmetric version of such ring expansion difficult. In the present work, a gold(I)-catalyzed asymmetric cyclopropanation/C–C cleavage/Wagner–Meerwein rearrangement of easily affordable yne-methylenecyclopropanes (1,6-yne-MCPs) has been developed to synthesize 3-azabicyclo[5.2.0]nonadiene, a bicyclic 7/4 ring (azepine fused with cyclobutane) with a bridgehead aryl substituent. This reaction overcomes the challenging loss of chirality from the Wagner–Meerwein rearrangement. Density functional theory calculations indicate that the chirality of the final product comes from the first cyclopropanation step in this reaction. The chirality in the resultant cyclopropane is lost in the following C–C cleavage step, generating rigid, planar cyclopropylmethyl carbocation intermediate. Then, only one carbon of the cyclopropyl group in the cyclopropylmethyl carbocation intermediate can migrate via ring expansion in the Wagner–Meerwein rearrangement process, and consequently, the chirality in the cyclopropane generated in the first step is transferred to the final product.
中文翻译:

涉及环丙烷化/ CC裂解/ Wagner-Meerwein重排的Yne-亚甲基环丙烷的不对称合成ze庚环酮
通过Wagner-Meerwein重排将原位生成的环丙基甲基阳离子扩环成环丁烷已广泛用于合成中。然而,产生的环丙基甲基阳离子是平面的,在手性前体的情况下,这将导致手性信息的损失,使得这种环膨胀的不对称形式变得困难。在目前的工作中,已经开发了金(I)催化的不对称环丙烷化/ CC裂解/瓦格纳-梅尔文重排的易负担的炔-亚甲基环丙烷(1,6-炔-MCP),以合成3-氮杂双环[5.2]。 0]壬二烯,具有桥头芳基取代基的双环7/4环(氮杂环丁烷与环丁烷稠合)。该反应克服了Wagner-Meerwein重排引起的手性丧失的挑战。密度泛函理论计算表明,最终产物的手性来自该反应的第一个环丙烷化步骤。在随后的CC裂解步骤中,生成的环丙烷中的手性消失,生成刚性的平面环丙基甲基碳正离子中间体。然后,环丙基甲基碳正离子中间体中只有一个环丙基碳原子可以通过Wagner-Meerwein重排过程中的扩环迁移,因此,第一步生成的环丙烷中的手性转移到了最终产物中。
更新日期:2019-07-26
中文翻译:

涉及环丙烷化/ CC裂解/ Wagner-Meerwein重排的Yne-亚甲基环丙烷的不对称合成ze庚环酮
通过Wagner-Meerwein重排将原位生成的环丙基甲基阳离子扩环成环丁烷已广泛用于合成中。然而,产生的环丙基甲基阳离子是平面的,在手性前体的情况下,这将导致手性信息的损失,使得这种环膨胀的不对称形式变得困难。在目前的工作中,已经开发了金(I)催化的不对称环丙烷化/ CC裂解/瓦格纳-梅尔文重排的易负担的炔-亚甲基环丙烷(1,6-炔-MCP),以合成3-氮杂双环[5.2]。 0]壬二烯,具有桥头芳基取代基的双环7/4环(氮杂环丁烷与环丁烷稠合)。该反应克服了Wagner-Meerwein重排引起的手性丧失的挑战。密度泛函理论计算表明,最终产物的手性来自该反应的第一个环丙烷化步骤。在随后的CC裂解步骤中,生成的环丙烷中的手性消失,生成刚性的平面环丙基甲基碳正离子中间体。然后,环丙基甲基碳正离子中间体中只有一个环丙基碳原子可以通过Wagner-Meerwein重排过程中的扩环迁移,因此,第一步生成的环丙烷中的手性转移到了最终产物中。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号