当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Protecting Group-Directed Diastereodivergent Synthesis of Chiral Tetrahydronaphthalene-Fused Spirooxindoles via Bifunctional Tertiary Amine Catalysis
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-07-26 00:00:00 , DOI: 10.1021/acs.joc.9b01501 Biao Wang 1 , Xiao-Hui Wang 2 , Wei Huang 1 , Jin Zhou 1 , Hong-Ping Zhu 1 , Cheng Peng 1 , Bo Han 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-07-26 00:00:00 , DOI: 10.1021/acs.joc.9b01501 Biao Wang 1 , Xiao-Hui Wang 2 , Wei Huang 1 , Jin Zhou 1 , Hong-Ping Zhu 1 , Cheng Peng 1 , Bo Han 1
Affiliation
|
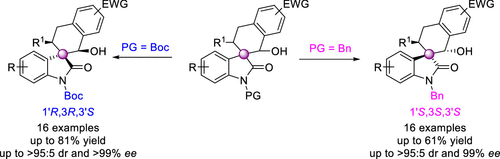
A collection of chiral spirocyclic tetrahydronaphthalene (THN)–oxindole hybrids bearing a quaternary carbon center at the β-position of THN has been developed. The diastereodivergent direct catalytic Michael–aldol reaction between 3-ylideneoxindole and 2-methylbenzaldehyde was accomplished by using bifunctional tertiary amine. Simply changing the protecting group on the substrate in the organocatalytic cascade reaction led to inverted diastereoselectivity in good yields with a high ee value. To explain the diastereodivergence of the organocatalytic Michael–aldol cascade, we also proposed plausible transition-state models for [4 + 2] annulation based on the observed stereochemistry of the products.
中文翻译:

双官能叔胺催化的保护基定向非对映异构性的手性四氢萘型螺氧杂吲哚合成
已经开发了在THN的β位带有一个季碳中心的手性螺环四氢萘(THN)-羟吲哚杂化物的集合。通过使用双官能叔胺完成3-亚硝基氧吲哚和2-甲基苯甲醛之间的非对映异构直接催化Michael-aldol反应。在有机催化级联反应中简单地改变底物上的保护基团就可以以高收率和高ee值实现非对映选择性的反向转化。为了解释有机催化迈克尔-阿尔多级联反应的非对映发散性,我们还基于观察到的产物立体化学,为[4 + 2]环化提出了合理的过渡态模型。
更新日期:2019-07-26
中文翻译:

双官能叔胺催化的保护基定向非对映异构性的手性四氢萘型螺氧杂吲哚合成
已经开发了在THN的β位带有一个季碳中心的手性螺环四氢萘(THN)-羟吲哚杂化物的集合。通过使用双官能叔胺完成3-亚硝基氧吲哚和2-甲基苯甲醛之间的非对映异构直接催化Michael-aldol反应。在有机催化级联反应中简单地改变底物上的保护基团就可以以高收率和高ee值实现非对映选择性的反向转化。为了解释有机催化迈克尔-阿尔多级联反应的非对映发散性,我们还基于观察到的产物立体化学,为[4 + 2]环化提出了合理的过渡态模型。















































 京公网安备 11010802027423号
京公网安备 11010802027423号