当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron(III) Speciation Observed at Aqueous and Glycerol Surfaces: Vibrational Sum Frequency and X-Ray
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-07-26 , DOI: 10.1021/jacs.9b05231 Lu Lin 1 , Jakub Husek 1 , Somnath Biswas 1 , Stephen M Baumler 1 , Tehseen Adel 1 , Ka Chon Ng 1 , L Robert Baker 1 , Heather C Allen 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-07-26 , DOI: 10.1021/jacs.9b05231 Lu Lin 1 , Jakub Husek 1 , Somnath Biswas 1 , Stephen M Baumler 1 , Tehseen Adel 1 , Ka Chon Ng 1 , L Robert Baker 1 , Heather C Allen 1
Affiliation
|
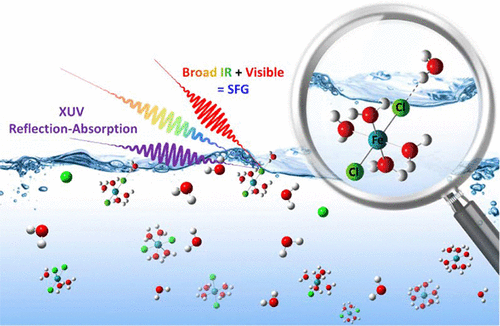
Aqueous solutions of FeCl3 have been widely studied to shed light on a number of processes from dissolution, mineralization, biology, electrocatalysis, corrosion, to microbial biomineralization. Yet there is little to no molecular level studies of the air-liquid FeCl3 interface. Here, both aqueous and glycerol FeCl3 solution surfaces are investigated with polarized vibrational sum frequency generation (SFG) spectroscopy. We also present the first ever extreme ultraviolet reflection-absorption (XUV-RA) spectroscopy measurements of solvated ions and complexes at a solution interface, and observe with both X-Ray photoelectron spectroscopy (XPS) and XUV-RA, existence of Fe(III) at the surface and in the near surface regions of glycerol FeCl3 solutions, where glycerol is used as a high vacuum compatible proxy for water. XPS showed Cl- and Fe(III) species with significant Fe(III) interfacial enrichment. In aqueous solutions, an electrical double layer (EDL) of Cl- and Fe(III) species at 0.5 molal FeCl3 concentration is observed as evidenced from an enhancement of molecular ordering of water dipoles, consistent with the observed behavior at the glycerol surface. At higher concentrations in water, the EDL appears to be substantially repressed indicative of further Fe(III) complex enrichment and dominance of a centrosymmetric Fe(III) species that is surface active. In addition, a significant vibrational red-shift of the dangling OH from the water molecules that straddle the air-water interface reveals that the second solvation shell of the surface active Fe(III) complex permeates the topmost layer of the aqueous interface.
中文翻译:

在水和甘油表面观察到的铁 (III) 形态:振动和频率和 X 射线
FeCl3 的水溶液已被广泛研究,以阐明从溶解、矿化、生物学、电催化、腐蚀到微生物生物矿化的许多过程。然而,几乎没有关于气液 FeCl3 界面的分子水平研究。在这里,水和甘油 FeCl3 溶液表面都用极化振动和频生成 (SFG) 光谱进行了研究。我们还首次对溶液界面处的溶剂化离子和络合物进行了极紫外反射吸收 (XUV-RA) 光谱测量,并用 X 射线光电子能谱 (XPS) 和 XUV-RA 观察了 Fe(III) 的存在) 在甘油 FeCl3 溶液的表面和近表面区域,其中甘油用作水的高真空兼容代理。XPS 显示 Cl-和 Fe(III) 物种具有显着的 Fe(III) 界面富集。在水溶液中,观察到浓度为 0.5 摩尔 FeCl3 的 Cl- 和 Fe(III) 物种的双电层 (EDL),这从水偶极子分子排序的增强中可以看出,这与在甘油表面观察到的行为一致。在水中浓度较高时,EDL 似乎受到显着抑制,表明具有表面活性的中心对称 Fe(III) 物质进一步富集 Fe(III) 复合物和占主导地位。此外,来自跨越空气-水界面的水分子的悬空 OH 的显着振动红移表明,表面活性 Fe(III) 复合物的第二溶剂化壳渗透到水界面的最上层。
更新日期:2019-07-26
中文翻译:

在水和甘油表面观察到的铁 (III) 形态:振动和频率和 X 射线
FeCl3 的水溶液已被广泛研究,以阐明从溶解、矿化、生物学、电催化、腐蚀到微生物生物矿化的许多过程。然而,几乎没有关于气液 FeCl3 界面的分子水平研究。在这里,水和甘油 FeCl3 溶液表面都用极化振动和频生成 (SFG) 光谱进行了研究。我们还首次对溶液界面处的溶剂化离子和络合物进行了极紫外反射吸收 (XUV-RA) 光谱测量,并用 X 射线光电子能谱 (XPS) 和 XUV-RA 观察了 Fe(III) 的存在) 在甘油 FeCl3 溶液的表面和近表面区域,其中甘油用作水的高真空兼容代理。XPS 显示 Cl-和 Fe(III) 物种具有显着的 Fe(III) 界面富集。在水溶液中,观察到浓度为 0.5 摩尔 FeCl3 的 Cl- 和 Fe(III) 物种的双电层 (EDL),这从水偶极子分子排序的增强中可以看出,这与在甘油表面观察到的行为一致。在水中浓度较高时,EDL 似乎受到显着抑制,表明具有表面活性的中心对称 Fe(III) 物质进一步富集 Fe(III) 复合物和占主导地位。此外,来自跨越空气-水界面的水分子的悬空 OH 的显着振动红移表明,表面活性 Fe(III) 复合物的第二溶剂化壳渗透到水界面的最上层。















































 京公网安备 11010802027423号
京公网安备 11010802027423号