当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Second Backbone: The Contribution of a Buried Asparagine Ladder to the Global and Local Stability of a Leucine-Rich Repeat Protein.
Biochemistry ( IF 2.9 ) Pub Date : 2019-08-06 , DOI: 10.1021/acs.biochem.9b00355 Sean A Klein 1 , Ananya Majumdar 2 , Doug Barrick 1
Biochemistry ( IF 2.9 ) Pub Date : 2019-08-06 , DOI: 10.1021/acs.biochem.9b00355 Sean A Klein 1 , Ananya Majumdar 2 , Doug Barrick 1
Affiliation
|
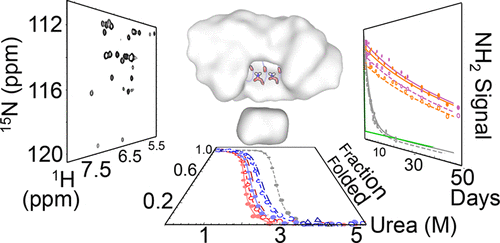
Parallel β-sheet-containing repeat proteins often display a structural motif in which conserved asparagines form a continuous ladder buried within the hydrophobic core. In such "asparagine ladders", the asparagine side-chain amides form a repetitive pattern of hydrogen bonds with neighboring main-chain NH and CO groups. Although asparagine ladders have been thought to be important for stability, there is little experimental evidence to support such speculation. Here we test the contribution of a minimal asparagine ladder from the leucine-rich repeat protein pp32 to stability and investigate lattice rigidity and hydrogen bond character using solution nuclear magnetic resonance (NMR) spectroscopy. Point substitutions of the two ladder asparagines of pp32 are strongly destabilizing and decrease the cooperativity of unfolding. The chemical shifts of the ladder side-chain HZ protons are shifted significantly downfield in the NMR spectrum and have low temperature coefficients, indicative of strong hydrogen bonding. In contrast, the HE protons are shifted upfield and have temperature coefficients close to zero, suggesting an asymmetry in hydrogen bond strength along the ladder. Ladder NH2 groups have weak 1H-15N cross-peak intensities; 1H-15N nuclear Overhauser effect and 15N CPMG experiments show this to be the result of high rigidity. Hydrogen exchange measurements demonstrate that the ladder NH2 groups exchange very slowly, with rates approaching the global exchange limit. Overall, these results show that the asparagine side chains are held in a very rigid, nondynamic structure, making a significant contribution to the overall stability. In this regard, buried asparagine ladders can be considered "second backbones" within the cores of their elongated β-sheet host proteins.
中文翻译:

第二个主干:埋藏的天冬酰胺梯对富含亮氨酸的重复蛋白的整体和局部稳定性的贡献。
含有平行β-折叠的重复蛋白通常表现出一个结构基序,其中保守的天冬酰胺形成埋在疏水核心内的连续梯子。在这种“天冬酰胺梯”中,天冬酰胺侧链酰胺与邻近的主链 NH 和 CO 基团形成重复的氢键模式。尽管天冬酰胺梯被认为对稳定性很重要,但几乎没有实验证据支持这种推测。在这里,我们测试了富含亮氨酸重复蛋白 pp32 的最小天冬酰胺梯对稳定性的贡献,并使用溶液核磁共振 (NMR) 光谱研究晶格刚性和氢键特征。 pp32 的两个天冬酰胺的点替换会强烈破坏稳定性并降低展开的协同性。梯形侧链 HZ 质子的化学位移在 NMR 谱中显着向低场移动,并且具有较低的温度系数,表明氢键作用较强。相比之下,HE 质子向高场移动,温度系数接近于零,表明氢键强度沿梯子不对称。梯形NH2基团具有较弱的1H-15N交叉峰强度; 1H-15N核奥豪塞效应和15N CPMG实验表明这是高刚性的结果。氢交换测量表明梯形 NH2 基团交换非常缓慢,速率接近全球交换极限。总的来说,这些结果表明天冬酰胺侧链保持在非常刚性的非动态结构中,对整体稳定性做出了重大贡献。在这方面,埋藏的天冬酰胺梯可以被认为是其伸长的β-片层宿主蛋白核心内的“第二主链”。
更新日期:2019-07-26
中文翻译:

第二个主干:埋藏的天冬酰胺梯对富含亮氨酸的重复蛋白的整体和局部稳定性的贡献。
含有平行β-折叠的重复蛋白通常表现出一个结构基序,其中保守的天冬酰胺形成埋在疏水核心内的连续梯子。在这种“天冬酰胺梯”中,天冬酰胺侧链酰胺与邻近的主链 NH 和 CO 基团形成重复的氢键模式。尽管天冬酰胺梯被认为对稳定性很重要,但几乎没有实验证据支持这种推测。在这里,我们测试了富含亮氨酸重复蛋白 pp32 的最小天冬酰胺梯对稳定性的贡献,并使用溶液核磁共振 (NMR) 光谱研究晶格刚性和氢键特征。 pp32 的两个天冬酰胺的点替换会强烈破坏稳定性并降低展开的协同性。梯形侧链 HZ 质子的化学位移在 NMR 谱中显着向低场移动,并且具有较低的温度系数,表明氢键作用较强。相比之下,HE 质子向高场移动,温度系数接近于零,表明氢键强度沿梯子不对称。梯形NH2基团具有较弱的1H-15N交叉峰强度; 1H-15N核奥豪塞效应和15N CPMG实验表明这是高刚性的结果。氢交换测量表明梯形 NH2 基团交换非常缓慢,速率接近全球交换极限。总的来说,这些结果表明天冬酰胺侧链保持在非常刚性的非动态结构中,对整体稳定性做出了重大贡献。在这方面,埋藏的天冬酰胺梯可以被认为是其伸长的β-片层宿主蛋白核心内的“第二主链”。

































 京公网安备 11010802027423号
京公网安备 11010802027423号