当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparisons of WO3 reduction to HxWO3 under thermochemical and electrochemical control†
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2019-07-25 , DOI: 10.1039/c9ta06394c
Evan V. Miu 1, 2, 3, 4 , James R. McKone 1, 2, 3, 4
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2019-07-25 , DOI: 10.1039/c9ta06394c
Evan V. Miu 1, 2, 3, 4 , James R. McKone 1, 2, 3, 4
Affiliation
|
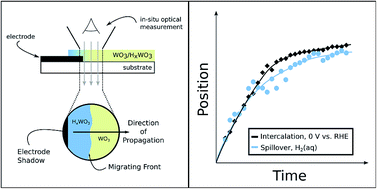
The use of renewable electricity as an energy source for the chemical industry is attractive from the standpoint of improved environmental sustainability. The development of such electrification strategies would greatly benefit from improved understanding of the relationships between thermochemical and electrochemical redox processes. We have used time-resolved optical microscopy to study HxWO3 bronze formation from WO3via thermochemical H-spillover and electrochemical H-intercalation reactions. The rates of hydrogen uptake in these films were found to be similar in each case, and too fast to be limited by proton diffusion in the solid state. These results point to a single predominant reaction mechanism that is primarily gated by interfacial electron-transfer rather than proton or hydrogen-atom diffusion.
中文翻译:

在热化学和电化学控制下 将WO 3还原为H x WO 3的比较†
从改善环境可持续性的角度来看,将可再生电力用作化学工业的能源是有吸引力的。对电气化策略的发展将极大地受益于对热化学和电化学氧化还原过程之间关系的更好理解。我们已经使用时间分辨光学显微镜研究了通过WO 3通过H 3形成的H x WO 3青铜。热化学H溢出和电化学H嵌入反应。发现这些膜中的氢吸收速率在每种情况下都是相似的,并且太快以至于不能受到固态中质子扩散的限制。这些结果表明主要由界面电子转移而非质子或氢原子扩散控制的单一主要反应机理。
更新日期:2019-10-23
中文翻译:

在热化学和电化学控制下 将WO 3还原为H x WO 3的比较†
从改善环境可持续性的角度来看,将可再生电力用作化学工业的能源是有吸引力的。对电气化策略的发展将极大地受益于对热化学和电化学氧化还原过程之间关系的更好理解。我们已经使用时间分辨光学显微镜研究了通过WO 3通过H 3形成的H x WO 3青铜。热化学H溢出和电化学H嵌入反应。发现这些膜中的氢吸收速率在每种情况下都是相似的,并且太快以至于不能受到固态中质子扩散的限制。这些结果表明主要由界面电子转移而非质子或氢原子扩散控制的单一主要反应机理。

































 京公网安备 11010802027423号
京公网安备 11010802027423号