当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeting the subpocket in xanthine oxidase: Design, synthesis, and biological evaluation of 2-[4-alkoxy-3-(1H-tetrazol-1-yl) phenyl]-6-oxo-1,6-dihydropyrimidine-5-carboxylic acid derivatives.
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-07-24 , DOI: 10.1016/j.ejmech.2019.07.062 Bing Zhang 1 , Xiwen Dai 1 , Ziyang Bao 1 , Qing Mao 1 , Yulin Duan 1 , Yuwei Yang 1 , Shaojie Wang 1
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-07-24 , DOI: 10.1016/j.ejmech.2019.07.062 Bing Zhang 1 , Xiwen Dai 1 , Ziyang Bao 1 , Qing Mao 1 , Yulin Duan 1 , Yuwei Yang 1 , Shaojie Wang 1
Affiliation
|
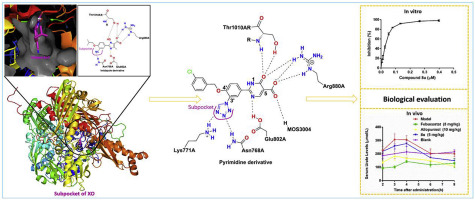
Xanthine oxidase is an important target for the treatment of hyperuricemia, gout and other related diseases. Analysis of the high-resolution structure of xanthine oxidase with febuxostat identified the existence of a subpocket formed by the residues Leu648, Asn768, Lys771, Leu1014 and Pro1076. In this study, we designed and synthesized a series of 2-[4-alkoxy-3-(1H-tetrazol-1-yl) phenyl]-6-oxo-1,6-dihydropyrimidine-5-carboxylic acid derivatives (8a-8z) with a tetrazole group targeting this subpocket of the xanthine oxidase active site, and they were further evaluated for their inhibitory potency against xanthine oxidase in vitro. The results showed that all the tested compounds (8a-8z) exhibited an apparent xanthine oxidase inhibitory potency, with IC50 values ranging from 0.0288 μM to 0.629 μM. Among them, compound 8u emerged as the most potent xanthine oxidase inhibitor, with an IC50 value of 0.0288 μM, which was comparable to febuxostat (IC50 = 0.0236 μM). The structure-activity relationship results revealed that the hydrophobic group at the 4'-position was indispensable for the inhibitory potency in vitro against xanthine oxidase. A Lineweaver-Burk plot revealed that the representative compound 8u acted as a mixed-type inhibitor for xanthine oxidase. Furthermore, molecular modeling studies were performed to gain insights into the binding mode of 8u with xanthine oxidase and suggested that the tetrazole group of the phenyl unit was accommodated in the subpocket, as expected. Moreover, a potassium oxonate-induced hyperuricemia model in rats was chosen to further confirm the hypouricemic effect of compound 8u, and the result demonstrated that compound 8u could effectively reduce serum uric acid levels at an oral dose of 5 mg/kg. In addition, acute oral toxicity study in mice indicated that compound 8u was nontoxic and tolerated at a dose up to 2000 mg/kg. Thus, compound 8u could be a potential and efficacious agent in treatment of hyperuricemia with low toxicity.
中文翻译:

针对黄嘌呤氧化酶中的子口袋:2- [4-烷氧基-3-(1H-四唑-1-基)苯基] -6-氧代-1,6-二氢嘧啶-5-羧酸的设计,合成和生物学评估衍生品。
黄嘌呤氧化酶是治疗高尿酸血症,痛风和其他相关疾病的重要靶标。用非布司他对黄嘌呤氧化酶的高分辨率结构进行分析,确定存在由残基Leu648,Ans768,Lys771,Leu1014和Pro1076形成的亚口袋。在这项研究中,我们设计并合成了一系列2- [4-烷氧基-3-(1H-四唑-1-基)苯基] -6-氧代-1,6-二氢嘧啶-5-羧酸衍生物(8a- 8z)具有靶向黄嘌呤氧化酶活性位点这一亚口袋的四唑基,并且进一步评估了它们在体外对黄嘌呤氧化酶的抑制能力。结果表明,所有测试的化合物(8a-8z)均表现出明显的黄嘌呤氧化酶抑制能力,IC50值为0.0288μM至0.629μM。他们之中,化合物8u成为最有效的黄嘌呤氧化酶抑制剂,IC50值为0.0288μM,与非布索坦相当(IC50 = 0.0236μM)。结构-活性关系结果表明,在体外对黄嘌呤氧化酶的抑制能力中,4'-位的疏水基团是必不可少的。Lineweaver-Burk图表明,代表性的化合物8u充当了黄嘌呤氧化酶的混合型抑制剂。此外,进行了分子建模研究,以深入了解8u与黄嘌呤氧化酶的结合模式,并表明,如预期的那样,亚苯基容纳了苯基单元的四唑基。此外,选择了由草酸钾诱导的大鼠高尿酸血症模型,以进一步确认化合物8u的降尿酸作用,结果表明,口服剂量为5 mg / kg时,化合物8u可以有效降低血清尿酸水平。此外,在小鼠中的急性口服毒性研究表明,化合物8u无毒,最高耐受剂量为2000 mg / kg。因此,化合物8u可能是低毒性高尿酸血症的潜在有效治疗剂。
更新日期:2019-07-24
中文翻译:

针对黄嘌呤氧化酶中的子口袋:2- [4-烷氧基-3-(1H-四唑-1-基)苯基] -6-氧代-1,6-二氢嘧啶-5-羧酸的设计,合成和生物学评估衍生品。
黄嘌呤氧化酶是治疗高尿酸血症,痛风和其他相关疾病的重要靶标。用非布司他对黄嘌呤氧化酶的高分辨率结构进行分析,确定存在由残基Leu648,Ans768,Lys771,Leu1014和Pro1076形成的亚口袋。在这项研究中,我们设计并合成了一系列2- [4-烷氧基-3-(1H-四唑-1-基)苯基] -6-氧代-1,6-二氢嘧啶-5-羧酸衍生物(8a- 8z)具有靶向黄嘌呤氧化酶活性位点这一亚口袋的四唑基,并且进一步评估了它们在体外对黄嘌呤氧化酶的抑制能力。结果表明,所有测试的化合物(8a-8z)均表现出明显的黄嘌呤氧化酶抑制能力,IC50值为0.0288μM至0.629μM。他们之中,化合物8u成为最有效的黄嘌呤氧化酶抑制剂,IC50值为0.0288μM,与非布索坦相当(IC50 = 0.0236μM)。结构-活性关系结果表明,在体外对黄嘌呤氧化酶的抑制能力中,4'-位的疏水基团是必不可少的。Lineweaver-Burk图表明,代表性的化合物8u充当了黄嘌呤氧化酶的混合型抑制剂。此外,进行了分子建模研究,以深入了解8u与黄嘌呤氧化酶的结合模式,并表明,如预期的那样,亚苯基容纳了苯基单元的四唑基。此外,选择了由草酸钾诱导的大鼠高尿酸血症模型,以进一步确认化合物8u的降尿酸作用,结果表明,口服剂量为5 mg / kg时,化合物8u可以有效降低血清尿酸水平。此外,在小鼠中的急性口服毒性研究表明,化合物8u无毒,最高耐受剂量为2000 mg / kg。因此,化合物8u可能是低毒性高尿酸血症的潜在有效治疗剂。































 京公网安备 11010802027423号
京公网安备 11010802027423号