当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Titanium Hydroxide Secondary Building Units in Metal-Organic Frameworks Catalyze Hydrogen Evolution under Visible Light
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-07-24 , DOI: 10.1021/jacs.9b05964 Yang Song 1 , Zhe Li 1, 2 , Yuanyuan Zhu 1 , Xuanyu Feng 1 , Justin S. Chen 1 , Michael Kaufmann 1 , Cheng Wang 2 , Wenbin Lin 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-07-24 , DOI: 10.1021/jacs.9b05964 Yang Song 1 , Zhe Li 1, 2 , Yuanyuan Zhu 1 , Xuanyu Feng 1 , Justin S. Chen 1 , Michael Kaufmann 1 , Cheng Wang 2 , Wenbin Lin 1
Affiliation
|
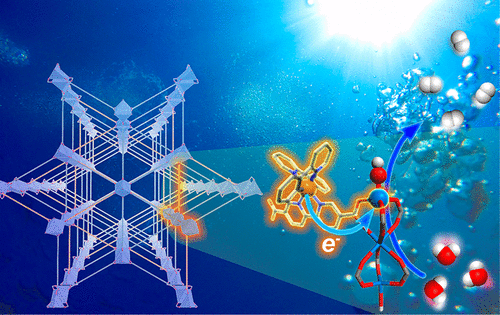
Herein we report the design of two new titanium metal-organic frameworks (MOFs), Ti3-BPDC-Ir and Ti3-BPDC-Ru, by doping [Ir(ppy)2(dcbpy)]Cl or [Ru(bpy)2(dcbpy)]Cl2 (bpy = 2,2'-bipyridine, ppy = 2-phenylpyridine, dcbpy = 2,2'-bipyridine-5,5'-dicarboxylate ) into the Ti3-BPDC framework (BPDC = biphenyl-4,4'-dicarboxylate). Hierarchical assembly of photosensitizing ligands and Ti3(OH)2 secondary building units (SBUs) facilitates multielectron transfer to drive photocatalytic hydrogen evolution (HER) under visible light with turnover numbers of 6632 and 786 for Ti3-BPDC-Ir and Ti3-BPDC-Ru, respectively. Photophysical and electrochemical studies establish the photocatalytic HER via reductive quenching of the excited photosensitizers followed by electron transfer from the reduced photosensitizers to Ti3(OH)2 SBUs and explain the catalytic difference between the two MOFs. Density functional theory calculations reveal key steps of HER via protonation of TiIII-OH to generate the TiIII species with a vacant coordination site followed by proton-coupled electron transfer to afford the key TiIV-H intermediate.
中文翻译:

金属-有机框架中的氢氧化钛二次构建单元在可见光下催化析氢
在此,我们报告了通过掺杂 [Ir(ppy)2(dcbpy)]Cl 或 [Ru(bpy)2( dcbpy)]Cl2(bpy = 2,2'-联吡啶,ppy = 2-苯基吡啶,dcbpy = 2,2'-bipyridine-5,5'-dicarboxylate)进入 Ti3-BPDC 框架(BPDC = biphenyl-4,4 '-二羧酸盐)。光敏配体和 Ti3(OH)2 二级构建单元 (SBU) 的分层组装促进多电子转移以驱动可见光下的光催化析氢 (HER),Ti3-BPDC-Ir 和 Ti3-BPDC-Ru 的转换数为 6632 和 786 , 分别。光物理和电化学研究通过激发光敏剂的还原猝灭和电子从还原的光敏剂转移到 Ti3(OH)2 SBU 来建立光催化 HER,并解释了两种 MOF 之间的催化差异。密度泛函理论计算揭示了 HER 的关键步骤,通过 TiIII-OH 质子化生成具有空配位点的 TiIII 物质,然后进行质子耦合电子转移以提供关键的 TiIV-H 中间体。
更新日期:2019-07-24
中文翻译:

金属-有机框架中的氢氧化钛二次构建单元在可见光下催化析氢
在此,我们报告了通过掺杂 [Ir(ppy)2(dcbpy)]Cl 或 [Ru(bpy)2( dcbpy)]Cl2(bpy = 2,2'-联吡啶,ppy = 2-苯基吡啶,dcbpy = 2,2'-bipyridine-5,5'-dicarboxylate)进入 Ti3-BPDC 框架(BPDC = biphenyl-4,4 '-二羧酸盐)。光敏配体和 Ti3(OH)2 二级构建单元 (SBU) 的分层组装促进多电子转移以驱动可见光下的光催化析氢 (HER),Ti3-BPDC-Ir 和 Ti3-BPDC-Ru 的转换数为 6632 和 786 , 分别。光物理和电化学研究通过激发光敏剂的还原猝灭和电子从还原的光敏剂转移到 Ti3(OH)2 SBU 来建立光催化 HER,并解释了两种 MOF 之间的催化差异。密度泛函理论计算揭示了 HER 的关键步骤,通过 TiIII-OH 质子化生成具有空配位点的 TiIII 物质,然后进行质子耦合电子转移以提供关键的 TiIV-H 中间体。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号