当前位置:
X-MOL 学术
›
Drug Test. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Replacing PAPS: In vitro phase II sulfation of steroids with the liver S9 fraction employing ATP and sodium sulfate
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2017-07-18 , DOI: 10.1002/dta.2224
Sumudu A. Weththasinghe 1 , Christopher C. Waller 1 , Han Ling Fam 1 , Bradley J. Stevenson 1 , Adam T. Cawley 2 , Malcolm D. McLeod 1
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2017-07-18 , DOI: 10.1002/dta.2224
Sumudu A. Weththasinghe 1 , Christopher C. Waller 1 , Han Ling Fam 1 , Bradley J. Stevenson 1 , Adam T. Cawley 2 , Malcolm D. McLeod 1
Affiliation
|
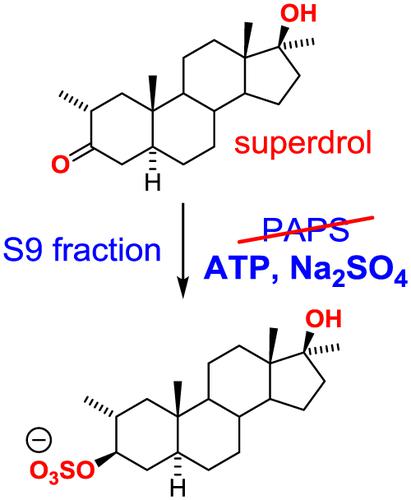
In vitro technologies provide the capacity to study drug metabolism where in vivo studies are precluded due to ethical or financial constraints. The metabolites generated by in vitro studies can assist anti‐doping laboratories to develop protocols for the detection of novel substances that would otherwise evade routine screening efforts. In addition, professional bodies such as the Association of Official Racing Chemists (AORC) currently permit the use of in‐vitro‐derived reference materials for confirmation purposes providing additional impetus for the development of cost effective in vitro metabolism platforms. In this work, alternative conditions for in vitro phase II sulfation using human, equine or canine liver S9 fraction were developed, with adenosine triphosphate (ATP) and sodium sulfate in place of the expensive and unstable co‐factor 3′‐phosphoadenosine‐5′‐phosphosulfate (PAPS), and employed for the generation of six representative steroidal sulfates. Using these conditions, the equine in vitro phase II metabolism of the synthetic or so‐called designer steroid furazadrol ([1′,2′]isoxazolo[4′,5′:2,3]‐5α‐androstan‐17β‐ol) was investigated, with ATP and Na2SO4 providing comparable metabolism to reactions using PAPS. The major in vitro metabolites of furazadrol matched those observed in a previously reported equine in vivo study. Finally, the equine in vitro phase II metabolism of the synthetic steroid superdrol (methasterone, 17β‐hydroxy‐2α,17α‐dimethyl‐5α‐androstan‐3‐one) was performed as a prediction of the in vivo metabolic profile.
中文翻译:

取代PAPS:使用ATP和硫酸钠,用肝脏S9馏分进行类固醇的体外II期硫酸盐化
体外技术提供了研究药物代谢的能力,其中由于伦理或财务上的限制而无法进行体内研究。体外研究产生的代谢物可以帮助反兴奋剂实验室制定检测新物质的方案,否则将避免常规的筛查工作。此外,诸如官方赛车化学家协会(AORC)之类的专业机构目前允许使用源自体外的参考材料进行确认,从而为开发具有成本效益的体外代谢平台提供了更多动力。在这项工作中,体外替代条件开发了使用人,马或犬肝S9馏分进行的II期硫酸盐化反应,用三磷酸腺苷(ATP)和硫酸钠代替昂贵且不稳定的辅助因子3'-磷酸腺苷-5'-磷酸硫酸盐(PAPS),并将其用于产生了六种代表性的甾族硫酸盐。在这些条件下,合成的或所谓的设计类固醇呋喃唑醇([1',2'] isoxazolo [4',5':2,3] ‐5α‐androstan‐17β‐ol)的马体外II期代谢对ATP和Na 2 SO 4的代谢提供了与使用PAPS的反应相当的代谢能力。呋喃唑醇的主要体外代谢物与先前报道的马体内研究中观察到的代谢物相符。最后,马合成类固醇超醇(美沙酮,17β-羟基-2α,17α-二甲基-5α-雄烷-3-酮)的体外II期代谢被用作体内代谢特征的预测。
更新日期:2017-07-18
中文翻译:

取代PAPS:使用ATP和硫酸钠,用肝脏S9馏分进行类固醇的体外II期硫酸盐化
体外技术提供了研究药物代谢的能力,其中由于伦理或财务上的限制而无法进行体内研究。体外研究产生的代谢物可以帮助反兴奋剂实验室制定检测新物质的方案,否则将避免常规的筛查工作。此外,诸如官方赛车化学家协会(AORC)之类的专业机构目前允许使用源自体外的参考材料进行确认,从而为开发具有成本效益的体外代谢平台提供了更多动力。在这项工作中,体外替代条件开发了使用人,马或犬肝S9馏分进行的II期硫酸盐化反应,用三磷酸腺苷(ATP)和硫酸钠代替昂贵且不稳定的辅助因子3'-磷酸腺苷-5'-磷酸硫酸盐(PAPS),并将其用于产生了六种代表性的甾族硫酸盐。在这些条件下,合成的或所谓的设计类固醇呋喃唑醇([1',2'] isoxazolo [4',5':2,3] ‐5α‐androstan‐17β‐ol)的马体外II期代谢对ATP和Na 2 SO 4的代谢提供了与使用PAPS的反应相当的代谢能力。呋喃唑醇的主要体外代谢物与先前报道的马体内研究中观察到的代谢物相符。最后,马合成类固醇超醇(美沙酮,17β-羟基-2α,17α-二甲基-5α-雄烷-3-酮)的体外II期代谢被用作体内代谢特征的预测。

































 京公网安备 11010802027423号
京公网安备 11010802027423号