Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Carfilzomib with immunomodulatory drugs for the treatment of newly diagnosed multiple myeloma.
Leukemia ( IF 12.8 ) Pub Date : 2019-07-24 , DOI: 10.1038/s41375-019-0517-6 Ola Landgren 1 , Pieter Sonneveld 2 , Andrzej Jakubowiak 3 , Mohamad Mohty 4 , Karim S Iskander 5 , Khalid Mezzi 5 , David S Siegel 6
Leukemia ( IF 12.8 ) Pub Date : 2019-07-24 , DOI: 10.1038/s41375-019-0517-6 Ola Landgren 1 , Pieter Sonneveld 2 , Andrzej Jakubowiak 3 , Mohamad Mohty 4 , Karim S Iskander 5 , Khalid Mezzi 5 , David S Siegel 6
Affiliation
|
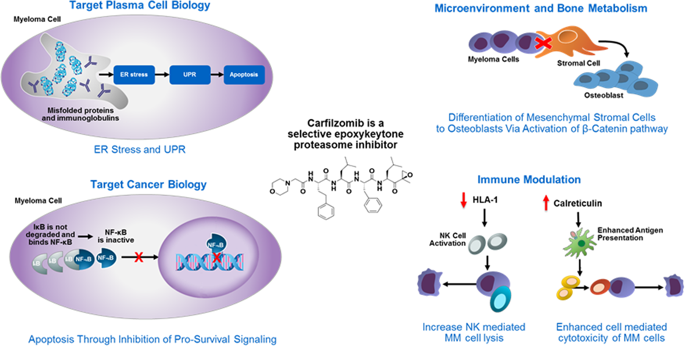
Carfilzomib, a selective proteasome inhibitor (PI), is approved for the treatment of patients with relapsed or refractory multiple myeloma (MM). Combination regimens incorporating a PI and immunomodulatory drug (IMiD) have been associated with deep responses and extended survival in patients with newly diagnosed MM (NDMM). Carfilzomib-based combinations with immunomodulators are being extensively studied in the frontline setting. The objective of this review was to describe efficacy and safety data for carfilzomib-based, PI/immunomodulatory combinations in NDMM. Information sources were articles indexed in PubMed and abstracts from key hematology/oncology congresses published between January 2012 and December 2018. PubMed and congresses were searched for prospective clinical studies assessing the combination of carfilzomib with an IMiD for NDMM treatment. Retrospective and preclinical reports, case reports/series, reviews, and clinical studies not evaluating carfilzomib-immunomodulator combinations in NDMM were excluded based on review of titles and abstracts. A total of nine articles and 72 abstracts were deemed relevant and included in the review. A total of six distinct carfilzomib-based, PI/immunomodulator combination regimens have been evaluated in 12 clinical trials. Overall, treatment with these regimens has resulted in deep responses, including high rates of negativity for minimal residual disease. These deep responses have translated to long progression-free survival and overall survival rates. Efficacy results for these regimens have generally been consistent across subgroups defined by age, transplant eligibility, and cytogenetic risk. The safety profile of carfilzomib in NDMM is consistent with that observed in the relapsed-refractory MM setting. Clinical studies have found that carfilzomib-based combinations with immunomodulators are highly active with a favorable safety profile in NDMM. The carfilzomib, lenalidomide, and dexamethasone (KRd) drug backbone is a promising foundation for treatment strategies aimed at achieving long-term, deep responses (functional cures) in the frontline setting. Several ongoing studies are evaluating KRd, with or without anti-CD38 monoclonal antibodies.
中文翻译:

卡非佐米与免疫调节药物一起治疗新诊断的多发性骨髓瘤。
卡非佐米是一种选择性蛋白酶体抑制剂(PI),被批准用于治疗复发或难治性多发性骨髓瘤(MM)患者。PI 和免疫调节药物 (IMiD) 的联合治疗方案与新诊断 MM (NDMM) 患者的深度缓解和延长生存期相关。基于卡非佐米与免疫调节剂的组合正在一线环境中得到广泛研究。本次综述的目的是描述基于卡非佐米的 PI/免疫调节组合在 NDMM 中的疗效和安全性数据。信息来源是 PubMed 中索引的文章以及 2012 年 1 月至 2018 年 12 月期间发表的重要血液学/肿瘤学大会的摘要。在 PubMed 和大会中检索了评估卡非佐米与 IMiD 联合治疗 NDMM 的前瞻性临床研究。根据标题和摘要的审查,排除了未评估 NDMM 中卡非佐米-免疫调节剂组合的回顾性和临床前报告、病例报告/系列、综述和临床研究。共有 9 篇文章和 72 篇摘要被认为相关并纳入审查。12 项临床试验对总共 6 种不同的基于卡非佐米的 PI/免疫调节剂组合方案进行了评估。总体而言,这些方案的治疗产生了深度反应,包括微小残留病的高阴性率。这些深层反应已转化为长期无进展生存率和总生存率。这些方案的疗效结果在按年龄、移植资格和细胞遗传学风险定义的亚组中总体上是一致的。卡非佐米在 NDMM 中的安全性与在复发难治性 MM 中观察到的安全性一致。临床研究发现,基于卡非佐米的免疫调节剂组合在 NDMM 中具有高度活性和良好的安全性。卡非佐米、来那度胺和地塞米松 (KRd) 药物主干是旨在在一线环境中实现长期、深度反应(功能性治愈)的治疗策略的有前景的基础。几项正在进行的研究正在评估 KRd,无论是否有抗 CD38 单克隆抗体。
更新日期:2019-07-24
中文翻译:

卡非佐米与免疫调节药物一起治疗新诊断的多发性骨髓瘤。
卡非佐米是一种选择性蛋白酶体抑制剂(PI),被批准用于治疗复发或难治性多发性骨髓瘤(MM)患者。PI 和免疫调节药物 (IMiD) 的联合治疗方案与新诊断 MM (NDMM) 患者的深度缓解和延长生存期相关。基于卡非佐米与免疫调节剂的组合正在一线环境中得到广泛研究。本次综述的目的是描述基于卡非佐米的 PI/免疫调节组合在 NDMM 中的疗效和安全性数据。信息来源是 PubMed 中索引的文章以及 2012 年 1 月至 2018 年 12 月期间发表的重要血液学/肿瘤学大会的摘要。在 PubMed 和大会中检索了评估卡非佐米与 IMiD 联合治疗 NDMM 的前瞻性临床研究。根据标题和摘要的审查,排除了未评估 NDMM 中卡非佐米-免疫调节剂组合的回顾性和临床前报告、病例报告/系列、综述和临床研究。共有 9 篇文章和 72 篇摘要被认为相关并纳入审查。12 项临床试验对总共 6 种不同的基于卡非佐米的 PI/免疫调节剂组合方案进行了评估。总体而言,这些方案的治疗产生了深度反应,包括微小残留病的高阴性率。这些深层反应已转化为长期无进展生存率和总生存率。这些方案的疗效结果在按年龄、移植资格和细胞遗传学风险定义的亚组中总体上是一致的。卡非佐米在 NDMM 中的安全性与在复发难治性 MM 中观察到的安全性一致。临床研究发现,基于卡非佐米的免疫调节剂组合在 NDMM 中具有高度活性和良好的安全性。卡非佐米、来那度胺和地塞米松 (KRd) 药物主干是旨在在一线环境中实现长期、深度反应(功能性治愈)的治疗策略的有前景的基础。几项正在进行的研究正在评估 KRd,无论是否有抗 CD38 单克隆抗体。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号