Environmental Pollution ( IF 7.6 ) Pub Date : 2019-07-23 , DOI: 10.1016/j.envpol.2019.07.118 Youming Dong , Minling Gao , Zhengguo Song , Weiwen Qiu
|
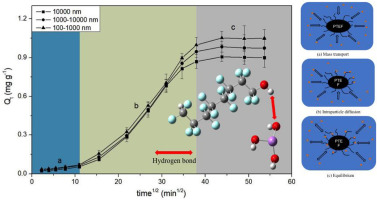
Microplastics exhibit active environmental behavior and unique surface characteristics, and act as carriers for the migration of trivalent arsenic (As(III)) in the environment. Herein, the mechanism by which polytetrafluoroethylene (PTFE) microplastic particles adsorb As(III) is systematically determined. The larger the size of PTFE particles, the smaller the specific surface area, the higher the point of zero charge (PZC), and the more unfavorable adsorption of As(III); the highest adsorption amount can reach 1.05 mg g−1. The adsorption process can be classified into three stages by the intraparticle diffusion model: external mass transfer, intraparticle diffusion, and dynamic equilibrium, of which the external mass transfer stage is the adsorption rate-limiting stage. The Langmuir model better represented the isotherm model and predict the adsorption results. The adsorption of As(III) by PTFE was an exothermic process, and because the increase in temperature broke the hydrogen bond, the amount of adsorption was decreased, which was not conducive to spontaneous adsorption. In the pH range of 3–7, as the pH value increased, the amount of As(III) adsorbed by PTFE gradually decreased, which may be related to the change in PZC for PTFE and the protonation of As(III). The H on the surface hydroxyl group of the PTFE exhibited a very large positive potential (+82.37 kcal mol−1). Thus, it can attract the arsenic oxyanion, and As(III) was subsequently adsorbed on the surface of the PTFE through the hydrogen bond on the hydroxyl group. Electrostatic force and non-covalent interaction were the key mechanisms affecting the PTFE adsorption.
中文翻译:

As(III)在不同尺寸的聚四氟乙烯颗粒上的吸附机理
微塑料表现出活跃的环境行为和独特的表面特性,并充当三价砷(As(III))在环境中迁移的载体。在本文中,系统地确定了聚四氟乙烯(PTFE)微塑料颗粒吸附As(III)的机理。PTFE颗粒的尺寸越大,比表面积越小,零电荷点(PZC)越高,As(III)的吸附越不利;最高吸附量可达到1.05 mg g -1。根据颗粒内扩散模型,吸附过程可分为三个阶段:外部传质,颗粒内扩散和动态平衡,其中外部传质阶段为吸附速率限制阶段。Langmuir模型可以更好地表示等温线模型并预测吸附结果。PTFE对As(III)的吸附是一个放热过程,由于温度升高会破坏氢键,因此吸附量减少,不利于自发吸附。在3-7的pH范围内,随着pH值的增加,PTFE吸附的As(III)的量逐渐减少,这可能与PTFE的PZC的变化和As(III)的质子化有关。PTFE表面羟基上的H表现出非常大的正电势(+82。-1)。因此,它可以吸引砷氧阴离子,随后通过羟基上的氢键将As(III)吸附在PTFE的表面上。静电力和非共价相互作用是影响PTFE吸附的关键机制。













































 京公网安备 11010802027423号
京公网安备 11010802027423号