当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Renal Excretion of Dabigatran: The Potential Role of Multidrug and Toxin Extrusion (MATE) Proteins.
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2019-08-07 , DOI: 10.1021/acs.molpharmaceut.9b00472 Hong Shen 1 , Ming Yao 1 , Michael Sinz 1 , Punit Marathe 1 , A David Rodrigues 1 , Mingshe Zhu 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2019-08-07 , DOI: 10.1021/acs.molpharmaceut.9b00472 Hong Shen 1 , Ming Yao 1 , Michael Sinz 1 , Punit Marathe 1 , A David Rodrigues 1 , Mingshe Zhu 1
Affiliation
|
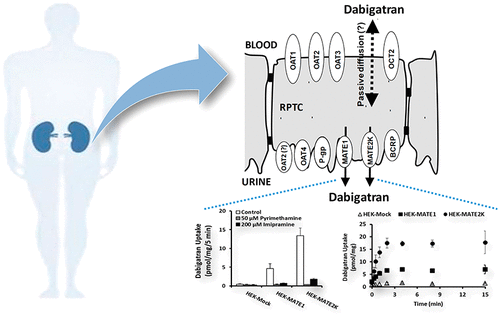
Following oral administration, dabigatran etexilate (DABE) is rapidly hydrolyzed to its active form, dabigatran. DABE, but not dabigatran, presents as a P-glycoprotein (P-gp) substrate and has increasingly been used as a probe drug. Therefore, although dosed as DABE, a P-gp drug-drug interaction (DDI) is reported as a dabigatran plasma concentration ratio (perpetrator versus placebo). Because the majority of a DABE dose (80 to 85%) is recovered in urine as unchanged dabigatran (renal active secretion is ∼25% of total clearance), dabigatran was evaluated in vitro as a substrate of various human renal transporters. Active (pyrimethamine-sensitive) dabigatran uptake was observed with human embryonic kidney (HEK) 293 cells expressing multidrug and toxin extrusion protein 1 (MATE1) and 2K (MATE2K), with Michaelis-Menten constant (Km) values of 4.0 and 8.0 μM, respectively. By comparison, no uptake of 2 μM dabigatran (versus mock-transfected HEK293 cells) was evident with HEK293 cells transfected with organic cation transporters (OCT1 and OCT2) and organic anion transporters (OAT1, 2, 3, and 4). The efflux ratios of dabigatran across P-gp- and BCRP (breast cancer resistance protein)-MDCK (Madin-Darby canine kidney) cell monolayers were 1.5 and 2.0 (versus mock-MDCK cell monolayers), suggesting dabigatran is a relatively poor P-gp and BCRP substrate. Three of five drugs (verapamil, ketoconazole, and quinidine) known to interact clinically with dabigatran, as P-gp inhibitors, presented as MATE inhibitors in vitro (IC50 = 1.0 to 25.2 μM). Taken together, although no basolateral transporter was identified for dabigatran, the results suggest that apical MATE1 and MATE2K could play an important role in its renal clearance. MATE-mediated renal secretion of dabigatran needs to be considered when interpreting the results of P-gp DDI studies following DABE administration.
中文翻译:

达比加群的肾脏排泄:多种药物和毒素挤出(MATE)蛋白的潜在作用。
口服后,达比加群酯(DABE)迅速水解成其活性形式达比加群。DABE,而不是达比加群,不是P-糖蛋白(P-gp)底物,并且已越来越多地用作探针药物。因此,尽管剂量为DABE,但据报道P-gp药物-药物相互作用(DDI)为达比加群血浆浓度比(犯罪者与安慰剂)。由于DABE剂量的大部分(80%至85%)是通过尿液中未改变的达比加群(肾脏活动性分泌物占总清除率的〜25%)而回收的,因此在体外评估达比加群作为各种人类肾脏转运蛋白的底物。在表达多种药物和毒素挤出蛋白1(MATE1)和2K(MATE2K)的人胚肾(HEK)293细胞中观察到活跃的(对乙胺嘧啶敏感的)达比加群吸收,Michaelis-Menten常数(Km)值为4。分别为0和8.0μM。相比之下,用有机阳离子转运蛋白(OCT1和OCT2)和有机阴离子转运蛋白(OAT1、2、3和4)转染的HEK293细胞没有吸收2μM达比加群(相对于模拟转染的HEK293细胞)。达比加群跨P-gp-和BCRP(乳腺癌抵抗蛋白)-MDCK(Madin-Darby犬肾)细胞单层的流出比分别为1.5和2.0(与模拟MDCK细胞单层相比),表明达比加群是相对较差的P- gp和BCRP底物。临床上已知与达比加群发生相互作用的五种药物中的三种(维拉帕米,酮康唑和奎尼丁)作为P-gp抑制剂在临床上与Mb抑制剂结合(IC50 = 1.0至25.2μM)。综上所述,尽管未发现达比加群的基底外侧转运蛋白,结果提示,顶端MATE1和MATE2K可能在其肾脏清除中起重要作用。在解释DABE给药后P-gp DDI研究的结果时,应考虑MATE介导的达比加群的肾脏分泌。
更新日期:2019-07-23
中文翻译:

达比加群的肾脏排泄:多种药物和毒素挤出(MATE)蛋白的潜在作用。
口服后,达比加群酯(DABE)迅速水解成其活性形式达比加群。DABE,而不是达比加群,不是P-糖蛋白(P-gp)底物,并且已越来越多地用作探针药物。因此,尽管剂量为DABE,但据报道P-gp药物-药物相互作用(DDI)为达比加群血浆浓度比(犯罪者与安慰剂)。由于DABE剂量的大部分(80%至85%)是通过尿液中未改变的达比加群(肾脏活动性分泌物占总清除率的〜25%)而回收的,因此在体外评估达比加群作为各种人类肾脏转运蛋白的底物。在表达多种药物和毒素挤出蛋白1(MATE1)和2K(MATE2K)的人胚肾(HEK)293细胞中观察到活跃的(对乙胺嘧啶敏感的)达比加群吸收,Michaelis-Menten常数(Km)值为4。分别为0和8.0μM。相比之下,用有机阳离子转运蛋白(OCT1和OCT2)和有机阴离子转运蛋白(OAT1、2、3和4)转染的HEK293细胞没有吸收2μM达比加群(相对于模拟转染的HEK293细胞)。达比加群跨P-gp-和BCRP(乳腺癌抵抗蛋白)-MDCK(Madin-Darby犬肾)细胞单层的流出比分别为1.5和2.0(与模拟MDCK细胞单层相比),表明达比加群是相对较差的P- gp和BCRP底物。临床上已知与达比加群发生相互作用的五种药物中的三种(维拉帕米,酮康唑和奎尼丁)作为P-gp抑制剂在临床上与Mb抑制剂结合(IC50 = 1.0至25.2μM)。综上所述,尽管未发现达比加群的基底外侧转运蛋白,结果提示,顶端MATE1和MATE2K可能在其肾脏清除中起重要作用。在解释DABE给药后P-gp DDI研究的结果时,应考虑MATE介导的达比加群的肾脏分泌。













































 京公网安备 11010802027423号
京公网安备 11010802027423号