当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis and biological evaluation of 2-(4-alkoxy-3-cyano)phenyl-6-oxo-1,6-dihydropyrimidine-5-carboxylic acid derivatives as novel xanthine oxidase inhibitors.
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-07-23 , DOI: 10.1016/j.ejmech.2019.07.061 Qing Mao 1 , Xiwen Dai 1 , Gaoyang Xu 2 , Yu Su 2 , Bing Zhang 1 , Dan Liu 1 , Shaojie Wang 1
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-07-23 , DOI: 10.1016/j.ejmech.2019.07.061 Qing Mao 1 , Xiwen Dai 1 , Gaoyang Xu 2 , Yu Su 2 , Bing Zhang 1 , Dan Liu 1 , Shaojie Wang 1
Affiliation
|
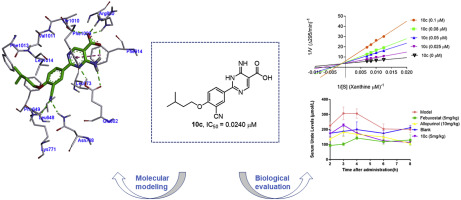
In our previous study, we reported a series of 1-hydroxy-2-phenyl-1H-imidazole-5-carboxylic acid derivatives that presented excellent in vitro xanthine oxidase (XO) inhibitory potency. To further investigate the structure-activity relationships of these compounds, the imidazole ring was transformed to a pyrimidine ring to design 2-(4-alkoxy-3-cyano)phenyl-6-oxo-1,6-dihydropyrimidine-5-carboxylic acids (8a-8j), 2-(4-alkoxy-3-cyano)phenyl-4-methyl-6-oxo-1,6-dihydropyrimidine-5-carboxylic acids (9c, 9e, 9j, 9l) and 2-(4-alkoxy-3-cyano)phenyl-6-imino-1,6-dihydropyrimidine-5-carboxylic acids (10c, 10e, 10j, 10l). These compounds exhibited remarkable in vitro XO inhibitory potency with IC50 values ranging from 0.0181 μM to 0.5677 μM. Specifically, compounds 10c and 10e, with IC50 values of 0.0240 μM and 0.0181 μM, respectively, emerged as the most potent XO inhibitors, and their potencies were comparable to that of febuxostat. Structure-activity relationship analysis revealed that the methyl group at 4-position of pyrimidine ring could damage the potency, and the XO inhibitory potency was maintained when carbonyl group was changed to an imino group. Lineweaver-Burk plot analysis revealed that the representative compound 10c acted as a mixed-type inhibitor. A potassium oxonate induced hyperuricemia model in rats was chosen to further confirm the hypouricemic effect of compound 10c, and the results showed that compound 10c (5 mg/kg) was able to significantly lower the serum uric acid level. Furthermore, in acute oral toxicity study, no sign of toxicity was observed when the mice were administered with a single 2000 mg/kg oral dose of compound 10c. These results suggested that compound 10c was a potent and promising uric acid-lowing agent for the treatment of hyperuricemia.
中文翻译:

作为新型黄嘌呤氧化酶抑制剂的2-(4-烷氧基-3-氰基)苯基-6-氧代-1,6-二氢嘧啶-5-羧酸衍生物的设计,合成和生物学评估。
在我们以前的研究中,我们报道了一系列1-羟基-2-苯基-1H-咪唑-5-羧酸衍生物,它们具有出色的体外黄嘌呤氧化酶(XO)抑制能力。为了进一步研究这些化合物的结构-活性关系,将咪唑环转化为嘧啶环,以设计2-(4-烷氧基-3-氰基)苯基-6-氧代1,6-二氢嘧啶-5-羧酸(8a-8j),2-(4-烷氧基-3-氰基)苯基-4-甲基-6-氧代-1,6-二氢嘧啶-5-羧酸(9c,9e,9j,9l)和2-( 4-烷氧基-3-氰基)苯基-6-亚氨基-1,6-二氢嘧啶-5-羧酸(10c,10e,10j,10l)。这些化合物具有出色的体外XO抑制能力,IC50值范围为0.0181μM至0.5677μM。具体而言,化合物10c和10e的IC50值分别为0.0240μM和0.0181μM,成为最有效的XO抑制剂,其效价与非布索坦相当。结构-活性关系分析表明,嘧啶环的4位甲基可能会破坏该效能,而当羰基变为亚氨基时,XO抑制效能得以维持。Lineweaver-Burk图分析表明,代表性化合物10c充当了混合型抑制剂。选择草酸钾诱导的大鼠高尿酸血症模型进一步证实化合物10c的降尿酸作用,结果表明化合物10c(5 mg / kg)能够显着降低血清尿酸水平。此外,在急性口服毒性研究中,当给小鼠口服单次口服剂量为2000 mg / kg的化合物10c时,未观察到毒性迹象。
更新日期:2019-07-23
中文翻译:

作为新型黄嘌呤氧化酶抑制剂的2-(4-烷氧基-3-氰基)苯基-6-氧代-1,6-二氢嘧啶-5-羧酸衍生物的设计,合成和生物学评估。
在我们以前的研究中,我们报道了一系列1-羟基-2-苯基-1H-咪唑-5-羧酸衍生物,它们具有出色的体外黄嘌呤氧化酶(XO)抑制能力。为了进一步研究这些化合物的结构-活性关系,将咪唑环转化为嘧啶环,以设计2-(4-烷氧基-3-氰基)苯基-6-氧代1,6-二氢嘧啶-5-羧酸(8a-8j),2-(4-烷氧基-3-氰基)苯基-4-甲基-6-氧代-1,6-二氢嘧啶-5-羧酸(9c,9e,9j,9l)和2-( 4-烷氧基-3-氰基)苯基-6-亚氨基-1,6-二氢嘧啶-5-羧酸(10c,10e,10j,10l)。这些化合物具有出色的体外XO抑制能力,IC50值范围为0.0181μM至0.5677μM。具体而言,化合物10c和10e的IC50值分别为0.0240μM和0.0181μM,成为最有效的XO抑制剂,其效价与非布索坦相当。结构-活性关系分析表明,嘧啶环的4位甲基可能会破坏该效能,而当羰基变为亚氨基时,XO抑制效能得以维持。Lineweaver-Burk图分析表明,代表性化合物10c充当了混合型抑制剂。选择草酸钾诱导的大鼠高尿酸血症模型进一步证实化合物10c的降尿酸作用,结果表明化合物10c(5 mg / kg)能够显着降低血清尿酸水平。此外,在急性口服毒性研究中,当给小鼠口服单次口服剂量为2000 mg / kg的化合物10c时,未观察到毒性迹象。






























 京公网安备 11010802027423号
京公网安备 11010802027423号