Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phase Separation in Supramolecular Hydrogels Based on Peptide Self-Assembly from Enzyme-Coated Nanoparticles.
Langmuir ( IF 3.7 ) Pub Date : 2019-07-23 00:00:00 , DOI: 10.1021/acs.langmuir.9b01420
Miryam Criado-Gonzalez 1, 2, 3 , Jennifer Rodon Fores 1 , Alain Carvalho 1 , Christian Blanck 1 , Marc Schmutz 1 , Leyla Kocgozlu 2, 3 , Pierre Schaaf 1, 2, 3 , Loïc Jierry 1 , Fouzia Boulmedais 1
Langmuir ( IF 3.7 ) Pub Date : 2019-07-23 00:00:00 , DOI: 10.1021/acs.langmuir.9b01420
Miryam Criado-Gonzalez 1, 2, 3 , Jennifer Rodon Fores 1 , Alain Carvalho 1 , Christian Blanck 1 , Marc Schmutz 1 , Leyla Kocgozlu 2, 3 , Pierre Schaaf 1, 2, 3 , Loïc Jierry 1 , Fouzia Boulmedais 1
Affiliation
|
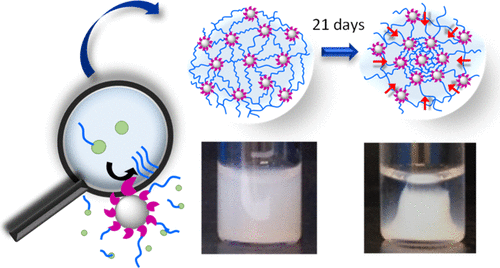
Spatial localization of biocatalysts, such as enzymes, has recently proven to be an effective process to direct supramolecular self-assemblies in a spatiotemporal way. In this work, silica nanoparticles (NPs) functionalized covalently by alkaline phosphatase ([email protected]) induce the localized growth of self-assembled peptide nanofibers from NPs by dephosphorylation of Fmoc–FFpY peptides (Fmoc: fluorenylmethyloxycarbonyl; F: phenylalanine; Y: tyrosine; p: phosphate group). The fibrillary nanoarchitecture around [email protected] underpins a homogeneous hydrogel, which unexpectedly undergoes a macroscopic shape change over time. This macroscopic change is due to a phase separation leading to a dense phase (in NPs and nanofibers) in the center of the vial and surrounded by a dilute one, which still contains NPs and peptide self-assemblies. We thus hypothesize that the phase separation is not a syneresis process. Such a change is only observed when the enzymes are localized on the NPs. The dense phase contracts with time until reaching a constant volume after several days. For a given phosphorylated peptide concentration, the dense phase contracts faster when the [email protected] concentration is increased. For a given [email protected] concentration, it condenses faster when the peptide concentration increases. We hypothesize that the appearance of a dense phase is not only due to attractive interactions between [email protected] but also to the strong interactions of self-assembled peptide nanofibers with the enzymes, covalently fixed on the NPs.
中文翻译:

超分子水凝胶中基于肽的自组装从酶涂层的纳米粒子中的相分离。
最近,已证明生物催化剂(例如酶)的空间定位是一种以时空方式指导超分子自组装的有效过程。在这项工作中,被碱性磷酸酶共价官能化的二氧化硅纳米颗粒(NP)(通过电子邮件保护)通过Fmoc–FF p Y肽(Fmoc:芴基甲氧基羰基; F:苯丙氨酸; Y:酪氨酸;p:磷酸基)。[受电子邮件保护的]周围的纤维状纳米结构支撑了均匀的水凝胶,该水凝胶出乎意料地随时间发生了宏观形状变化。这种宏观变化是由于相分离导致小瓶中心(在NPs和纳米纤维中)的致密相(被NPs和肽的自组装体稀释)所围绕,而该相在小瓶的中心并被稀释。因此,我们假设相分离不是脱水收缩过程。仅当酶位于NP上时才能观察到这种变化。稠密相随时间收缩,直到几天后达到恒定体积。对于给定的磷酸化肽浓度,当[电子邮件保护的]浓度增加时,致密相收缩更快。对于给定的[受电子邮件保护的]集中注意力,当肽浓度增加时,它的凝结速度更快。我们假设致密相的出现不仅是由于[受电子邮件保护]之间的有吸引力的相互作用,还归因于自组装肽纳米纤维与酶的强相互作用,这些酶共价固定在NPs上。
更新日期:2019-07-23
中文翻译:

超分子水凝胶中基于肽的自组装从酶涂层的纳米粒子中的相分离。
最近,已证明生物催化剂(例如酶)的空间定位是一种以时空方式指导超分子自组装的有效过程。在这项工作中,被碱性磷酸酶共价官能化的二氧化硅纳米颗粒(NP)(通过电子邮件保护)通过Fmoc–FF p Y肽(Fmoc:芴基甲氧基羰基; F:苯丙氨酸; Y:酪氨酸;p:磷酸基)。[受电子邮件保护的]周围的纤维状纳米结构支撑了均匀的水凝胶,该水凝胶出乎意料地随时间发生了宏观形状变化。这种宏观变化是由于相分离导致小瓶中心(在NPs和纳米纤维中)的致密相(被NPs和肽的自组装体稀释)所围绕,而该相在小瓶的中心并被稀释。因此,我们假设相分离不是脱水收缩过程。仅当酶位于NP上时才能观察到这种变化。稠密相随时间收缩,直到几天后达到恒定体积。对于给定的磷酸化肽浓度,当[电子邮件保护的]浓度增加时,致密相收缩更快。对于给定的[受电子邮件保护的]集中注意力,当肽浓度增加时,它的凝结速度更快。我们假设致密相的出现不仅是由于[受电子邮件保护]之间的有吸引力的相互作用,还归因于自组装肽纳米纤维与酶的强相互作用,这些酶共价固定在NPs上。































 京公网安备 11010802027423号
京公网安备 11010802027423号