当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical Thermoelectric Conversion with Polysulfide as Redox Species.
ChemSusChem ( IF 7.5 ) Pub Date : 2019-08-07 , DOI: 10.1002/cssc.201901566 Yimin Liang 1 , Joseph K-H Hui 1 , Teppei Yamada 1, 2 , Nobuo Kimizuka 1, 2
ChemSusChem ( IF 7.5 ) Pub Date : 2019-08-07 , DOI: 10.1002/cssc.201901566 Yimin Liang 1 , Joseph K-H Hui 1 , Teppei Yamada 1, 2 , Nobuo Kimizuka 1, 2
Affiliation
|
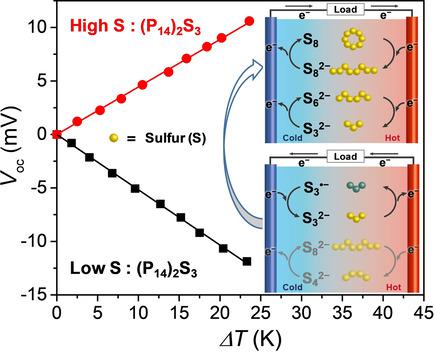
Thermocells convert waste heat to electricity without any pollution; however, the high cost and corrosivity of redox species hinder their commercialization. In this work, a thermocell that utilizes abundant polysulfide as redox species was demonstrated for the first time. 1-Butyl-1-methylpyrrolidinium polysulfide [(P14 )2 S3 ] was synthesized, and the redox species were prepared by the addition of sulfur to the (P14 )2 S3 solution in DMSO. In thermoelectric measurements, the Seebeck coefficient changed from -0.68 to +0.5 mV K-1 through addition of sulfur to the cell. Operando UV/Vis spectroscopy and open-circuit voltage analysis revealed that this effect was attributed to the change in the dominating redox reactions by the addition of sulfur. This result also provides a thermodynamic view on polysulfides electrochemistry, which is of high importance for lithium-sulfur batteries.
中文翻译:

用多硫化物作为氧化还原物质进行电化学热电转化。
热电池将废热转化为电能而没有任何污染;然而,氧化还原物质的高成本和腐蚀性阻碍了它们的商业化。在这项工作中,首次展示了利用丰富的多硫化物作为氧化还原物质的热电池。合成了1-丁基-1-甲基吡咯烷鎓多硫化物[(P14)2 S3],通过向DMSO中的(P14)2 S3溶液中添加硫来制备氧化还原物质。在热电测量中,通过向池中添加硫,塞贝克系数从-0.68变为+0.5 mV K-1。Operando紫外/可见光谱和开路电压分析表明,这种影响归因于添加硫引起的主要氧化还原反应的变化。该结果还提供了有关多硫化物电化学的热力学观点,
更新日期:2019-08-07
中文翻译:

用多硫化物作为氧化还原物质进行电化学热电转化。
热电池将废热转化为电能而没有任何污染;然而,氧化还原物质的高成本和腐蚀性阻碍了它们的商业化。在这项工作中,首次展示了利用丰富的多硫化物作为氧化还原物质的热电池。合成了1-丁基-1-甲基吡咯烷鎓多硫化物[(P14)2 S3],通过向DMSO中的(P14)2 S3溶液中添加硫来制备氧化还原物质。在热电测量中,通过向池中添加硫,塞贝克系数从-0.68变为+0.5 mV K-1。Operando紫外/可见光谱和开路电压分析表明,这种影响归因于添加硫引起的主要氧化还原反应的变化。该结果还提供了有关多硫化物电化学的热力学观点,





















































 京公网安备 11010802027423号
京公网安备 11010802027423号