当前位置:
X-MOL 学术
›
ACS Appl. Nano Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aerobic Oxidation of Alcohols over Isolated Single Au Atoms Supported on CeO2 Nanorods: Catalysis of Interfacial [O–Ov–Ce–O–Au] Sites
ACS Applied Nano Materials ( IF 5.3 ) Pub Date : 2019-08-05 , DOI: 10.1021/acsanm.9b01091 Lijun Lei 1, 2 , Huan Liu 1, 2 , Zhiwei Wu 1 , Zhangfeng Qin 1 , Guofu Wang 1 , Jingyuan Ma 3 , Li Luo 1, 2 , Weibin Fan 1 , Jianguo Wang 1, 2
ACS Applied Nano Materials ( IF 5.3 ) Pub Date : 2019-08-05 , DOI: 10.1021/acsanm.9b01091 Lijun Lei 1, 2 , Huan Liu 1, 2 , Zhiwei Wu 1 , Zhangfeng Qin 1 , Guofu Wang 1 , Jingyuan Ma 3 , Li Luo 1, 2 , Weibin Fan 1 , Jianguo Wang 1, 2
Affiliation
|
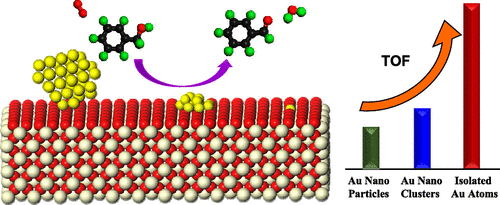
The oxidation of alcohols is a kind of very important reaction in industry and has caused great concern in recent years, especially with molecular oxygen as the oxidant. Among various catalysts reported in the literature, Au/CeO2 exhibits excellent catalytic performance in the oxidation of alcohols, with aldehydes as the target product. However, there is still not a clear consensus regarding the nature of the active sites of Au/CeO2 related catalysts. In this contribution, three distinctly different Au species supported on CeO2 nanorods (CeO2-NR) were prepared (viz., isolated single Au atoms (Au-SA), Au nanoclusters (Au-NC), and Au nanoparticles (Au-NP)); their catalytic performances in the aerobic oxidation of alcohols was comparatively investigated in order to discriminate the active sites in Au/CeO2. A series of characterization results combining with DFT calculation illustrates that the performance of Au/CeO2 catalysts in alcohol oxidation is closely related to the size of Au species and the concentration of oxygen vacancies (Ov), where the interfacial [O–Ov–Ce–O–Au] sites between Au species and CeO2 support play an important role in catalyzing the alcohol oxidation. The oxygen vacancies in CeO2 facilitate the adsorption of alcohol and the dissociation of the O–H bond, whereas the adjacent Au3+/Au+ species can promote the elimination of β-hydride in the alkoxide intermediate. As the Au-SA/CeO2-NR catalyst is provided with abundant active [O–Ov–Ce–O–Au] sites, it exhibits much higher activity in the oxidation of alcohols, in comparison with Au-NC/CeO2-NR and Au-NP/CeO2-NR. The insight shown in this work should be helpful in understanding the catalysis of interfacial sites between metal and an oxide support and developing better catalysts for the oxidation of alcohols.
中文翻译:

CeO2纳米棒支撑的单个金原子上的酒精有氧氧化:界面[O–Ov–Ce–O–Au]位点的催化
醇的氧化是工业上非常重要的一种反应,近年来引起了人们的广泛关注,特别是以分子氧作为氧化剂。在文献报道的各种催化剂中,Au / CeO 2在以醛为目标产物的醇的氧化中表现出优异的催化性能。然而,关于Au / CeO 2相关催化剂的活性位点的性质仍未达成明确共识。在此贡献中,三种明显不同的Au物种负载在CeO 2纳米棒上(CeO 2-NR)(即分离的单个Au原子(Au-SA),Au纳米团簇(Au-NC)和Au纳米颗粒(Au-NP)); 为了区分Au / CeO 2中的活性位点,对它们在醇的有氧氧化中的催化性能进行了比较研究。结合DFT计算的一系列表征结果表明,Au / CeO 2催化剂在醇氧化中的性能与Au种类的大小和氧空位(Ov)的浓度密切相关,其中界面[O–Ov–Ce Au物种和CeO 2载体之间的–O–Au]位点在催化醇氧化中起重要作用。CeO 2中的氧空位促进醇的吸附和O–H键的解离,而相邻的Au 3+ / Au +物种可以促进消除醇盐中间体中的β-氢化物。由于在Au-SA /的CeO 2 -NR催化剂设置有丰富的活性[O-OV-的CeO金]位点,其表现出高得多的活性在醇氧化,在用Au-NC /比较的CeO 2 - NR和Au-NP / CeO 2 -NR。这项工作中显示的见识应有助于理解金属与氧化物载体之间的界面部位的催化作用,并开发出更好的醇氧化催化剂。
更新日期:2019-08-05
中文翻译:

CeO2纳米棒支撑的单个金原子上的酒精有氧氧化:界面[O–Ov–Ce–O–Au]位点的催化
醇的氧化是工业上非常重要的一种反应,近年来引起了人们的广泛关注,特别是以分子氧作为氧化剂。在文献报道的各种催化剂中,Au / CeO 2在以醛为目标产物的醇的氧化中表现出优异的催化性能。然而,关于Au / CeO 2相关催化剂的活性位点的性质仍未达成明确共识。在此贡献中,三种明显不同的Au物种负载在CeO 2纳米棒上(CeO 2-NR)(即分离的单个Au原子(Au-SA),Au纳米团簇(Au-NC)和Au纳米颗粒(Au-NP)); 为了区分Au / CeO 2中的活性位点,对它们在醇的有氧氧化中的催化性能进行了比较研究。结合DFT计算的一系列表征结果表明,Au / CeO 2催化剂在醇氧化中的性能与Au种类的大小和氧空位(Ov)的浓度密切相关,其中界面[O–Ov–Ce Au物种和CeO 2载体之间的–O–Au]位点在催化醇氧化中起重要作用。CeO 2中的氧空位促进醇的吸附和O–H键的解离,而相邻的Au 3+ / Au +物种可以促进消除醇盐中间体中的β-氢化物。由于在Au-SA /的CeO 2 -NR催化剂设置有丰富的活性[O-OV-的CeO金]位点,其表现出高得多的活性在醇氧化,在用Au-NC /比较的CeO 2 - NR和Au-NP / CeO 2 -NR。这项工作中显示的见识应有助于理解金属与氧化物载体之间的界面部位的催化作用,并开发出更好的醇氧化催化剂。













































 京公网安备 11010802027423号
京公网安备 11010802027423号