Scientific Reports ( IF 3.8 ) Pub Date : 2019-07-23 , DOI: 10.1038/s41598-019-47063-1 Hiroshi Kawasaki 1 , Natsumi Soma 1 , Robert H Kretsinger 2
|
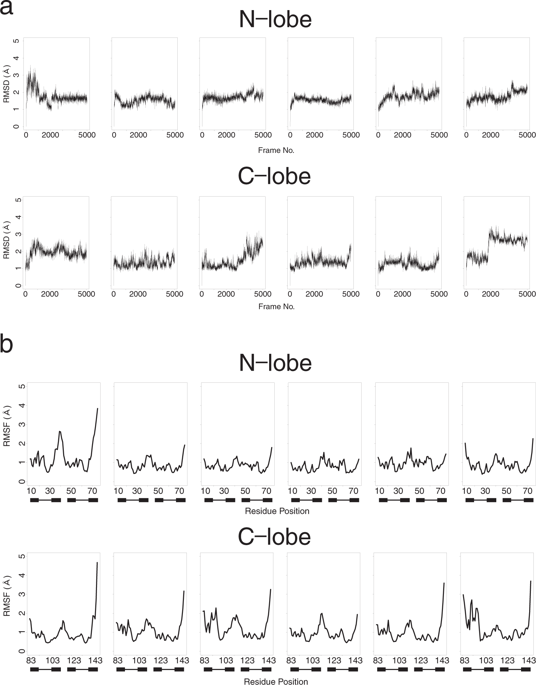
Calmodulin is a calcium binding protein with two lobes, N-lobe and C-lobe, which evolved from duplication and fusion of a single precursor lobe of a pair of EF-hand. These two lobes of calmodulin show subtle differences in calcium binding and target recognition; these are important for the functions of calmodulin. Since the structures, especially main chain conformations, of two EF-lobes in holo-form are quite similar; this is a good example to evaluate the effect of side chains for structural dynamics. We analyzed the structure of calmodulin using molecular dynamics and found differences in conformational ensembles between N- and C-lobes. We also showed the mutant structures created by homology modeling could reproduce the difference of dynamic motion between N- and C-lobes.
中文翻译:

钙结合和/或靶标识别的钙调蛋白构象变化的分子动力学研究。
钙调蛋白是一种钙结合蛋白,具有两个叶(N 叶和 C 叶),由一对 EF 手的单个前体叶的复制和融合进化而来。钙调蛋白的这两个叶在钙结合和目标识别方面表现出细微的差异。这些对于钙调蛋白的功能很重要。由于全息形式的两个EF叶的结构,特别是主链构象非常相似;这是评估侧链对结构动力学影响的一个很好的例子。我们利用分子动力学分析了钙调蛋白的结构,发现了 N 叶和 C 叶之间构象整体的差异。我们还表明,通过同源建模创建的突变结构可以重现 N 叶和 C 叶之间动态运动的差异。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号