Electrochimica Acta ( IF 5.5 ) Pub Date : 2019-07-23 , DOI: 10.1016/j.electacta.2019.134577 Xuehui Liu , Jian Wu
|
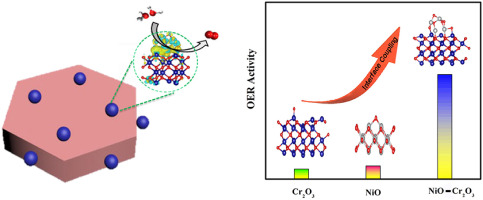
The oxygen evolution reaction is a key reaction in rechargeable metal air battery. Nickel oxide (NiO) nanoparticles are a potential low-cost oxygen evolution reaction (OER) electrocatalyst, and its catalytic activity and stability are still to be further improved. The construction of interfacial catalysts can improve their electrocatalytic activity. In this work, we report that nickel oxide (NiO) nanoclusters supported on chromium oxide (Cr2O3) nanosheets form a novel NiO–Cr2O3 heterostructure as an effective electrocatalyst for OER. Compared with pristine NiO and Cr2O3, NiO–Cr2O3 exhibits higher OER activity with smaller overpotential of 270 mV and lower Tafel slope of 30 mVdec−1, as well as durability under alkaline conditions. The X-ray photoelectron spectroscopy, In-situ Raman spectra and theoretical calculations reveal a source of high catalytic activity of NiO–Cr2O3. The results show that the activity site is located at Cr–Ni site of the NiO–Cr2O3. Due to the obvious charge transfer between Cr and Ni atoms at the interface, the change of Ni and Cr electronic structure reduces the adsorption energy of oxygen species and increases their catalytic activity. This work constructs a new Cr–Ni interface coupling method through NiO–Cr2O3 heterostructure, which provides a new strategy for designing new efficient and inexpensive OER catalysts.
中文翻译:

NiO-Cr 2 O 3异质结构的耦合界面结构,可有效地产生电催化氧
氧气析出反应是可充电金属空气电池中的关键反应。氧化镍(NiO)纳米粒子是潜在的低成本氧气析出反应(OER)电催化剂,其催化活性和稳定性仍有待进一步提高。界面催化剂的构造可以改善其电催化活性。在这项工作中,我们报道了负载在氧化铬(Cr 2 O 3)纳米片上的氧化镍(NiO)纳米团簇形成了一种新型的NiO-Cr 2 O 3异质结构,可以作为OER的有效电催化剂。与原始NiO和Cr 2 O 3相比,NiO–Cr 2 O 3表现出较高的OER活性,较小的270 mV过电势,较低的Tafel斜率30 mVdec -1,以及在碱性条件下的耐久性。X射线光电子能谱,原位拉曼光谱和理论计算揭示了NiO–Cr 2 O 3的高催化活性的来源。结果表明,活性位点位于NiO-Cr 2 O 3的Cr-Ni位点。由于界面上Cr和Ni原子之间明显的电荷转移,Ni和Cr电子结构的变化降低了氧种类的吸附能并增加了它们的催化活性。这项工作通过NiO-Cr 2 O 3构造了一种新的Cr-Ni界面偶联方法。 异质结构,为设计新型高效,廉价的OER催化剂提供了新的策略。































 京公网安备 11010802027423号
京公网安备 11010802027423号