当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure-guided development of purine amide, hydroxamate, and amidoxime for the inhibition of non-small cell lung cancer.
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-07-22 , DOI: 10.1016/j.ejmech.2019.07.054 Meng-Ruo Huang , Yuan-Ling Hsu , Tzu-Chen Lin , Ting-Jen Cheng , Ling-Wei Li , Yu-Wei Tseng , Yu-shu Chou , Jyung-Hurng Liu , Szu-Hua Pan , Jim-Min Fang , Chi-Huey Wong
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-07-22 , DOI: 10.1016/j.ejmech.2019.07.054 Meng-Ruo Huang , Yuan-Ling Hsu , Tzu-Chen Lin , Ting-Jen Cheng , Ling-Wei Li , Yu-Wei Tseng , Yu-shu Chou , Jyung-Hurng Liu , Szu-Hua Pan , Jim-Min Fang , Chi-Huey Wong
|
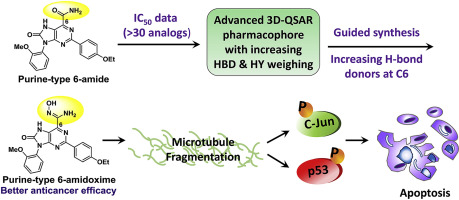
An 8-oxopurine-6-carboxamide compound (1a) was previously identified as an inhibitor of non-small cell lung cancer (NSCLC). In this study, more than 30 purine-6-carboxamide derivatives with variations at the C2, N7, C8, and N9 positions were synthesized to investigate the structure-activity relationship as a basis for the construction of an advanced pharmacophore model. This model suggests that purine-6-hydroxamate and purine-6-amidoxime analogs could form more hydrogen bonds with a target protein to enhance the inhibitory activities against H1975 cells. Among the series of analogs, hydroxamate 17 and amidoxime 19a exhibited excellent potency against H1975 cells (IC50 < 1.5 μM) and other lung cancer cells with either wild-type or mutated epidermal growth factor receptor (EGFR). Mouse experiments indicated that compounds 17 and 19a were efficient anticancer agents with no appreciable toxicity. The mechanisms of action for the induction of cell apoptosis were determined to involve microtubule fragmentation and p53-mediated signaling pathways.
中文翻译:

嘌呤酰胺,异羟肟酸酯和a胺肟对非小细胞肺癌抑制作用的结构指导开发。
先前已确定8-氧嘌呤-6-羧酰胺化合物(1a)是非小细胞肺癌(NSCLC)的抑制剂。在这项研究中,合成了30多个在C2,N7,C8和N9位置具有变化的嘌呤-6-羧酰胺衍生物,以研究其结构活性关系,以此为基础构建高级药效团模型。该模型表明嘌呤6-异羟肟酸酯和嘌呤6-d肟类似物可与靶蛋白形成更多的氢键,从而增强对H1975细胞的抑制活性。在一系列类似物中,异羟肟酸酯17和a胺肟19a对H1975细胞(IC50 <1.5μM)和其他具有野生型或突变表皮生长因子受体(EGFR)的肺癌细胞表现出出色的效力。小鼠实验表明,化合物17和19a是有效的抗癌药,没有明显的毒性。确定了诱导细胞凋亡的作用机制,涉及微管片段化和p53介导的信号通路。
更新日期:2019-07-22
中文翻译:

嘌呤酰胺,异羟肟酸酯和a胺肟对非小细胞肺癌抑制作用的结构指导开发。
先前已确定8-氧嘌呤-6-羧酰胺化合物(1a)是非小细胞肺癌(NSCLC)的抑制剂。在这项研究中,合成了30多个在C2,N7,C8和N9位置具有变化的嘌呤-6-羧酰胺衍生物,以研究其结构活性关系,以此为基础构建高级药效团模型。该模型表明嘌呤6-异羟肟酸酯和嘌呤6-d肟类似物可与靶蛋白形成更多的氢键,从而增强对H1975细胞的抑制活性。在一系列类似物中,异羟肟酸酯17和a胺肟19a对H1975细胞(IC50 <1.5μM)和其他具有野生型或突变表皮生长因子受体(EGFR)的肺癌细胞表现出出色的效力。小鼠实验表明,化合物17和19a是有效的抗癌药,没有明显的毒性。确定了诱导细胞凋亡的作用机制,涉及微管片段化和p53介导的信号通路。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号