当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Early Stages of Electrochemical Oxidation of Cu(111) and Polycrystalline Cu Surfaces Revealed by in situ Raman Spectroscopy
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-07-21 , DOI: 10.1021/jacs.9b04638 Nataraju Bodappa 1 , Min Su 1 , Yu Zhao 1 , Jia-Bo Le 1 , Wei-Min Yang 2 , Petar Radjenovic 1 , Jin-Chao Dong 1 , Jun Cheng 1 , Zhong-Qun Tian 1 , Jian-Feng Li 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-07-21 , DOI: 10.1021/jacs.9b04638 Nataraju Bodappa 1 , Min Su 1 , Yu Zhao 1 , Jia-Bo Le 1 , Wei-Min Yang 2 , Petar Radjenovic 1 , Jin-Chao Dong 1 , Jun Cheng 1 , Zhong-Qun Tian 1 , Jian-Feng Li 1, 2
Affiliation
|
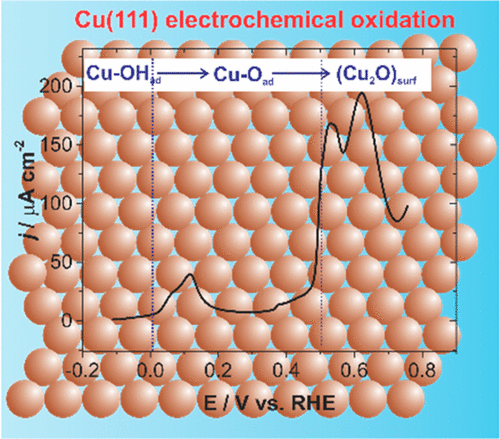
Investigating the chemical nature of the adsorbed intermediate species on well-defined Cu single crystal substrates is crucial in understanding many electrocatalytic reactions. Herein, we sys-tematically study the early stages of electrochemical oxidation of Cu(111) and polycrystalline Cu surfaces in different pH electro-lytes using in situ shell-isolated nanoparticle-enhanced Raman spectroscopy (SHINERS). On Cu(111), for the first time, we identified surface OH species which convert to chemisorbed "O" before forming Cu2O in alkaline (0.01 M KOH) and neutral (0.1 M Na2SO4) electrolytes; while at the Cu(poly) surface, we only detected the presence of surface hydroxide. Whereas, in a strong-ly acidic solution (0.1 M H2SO4), sulfate replaces the hydrox-yl/oxy species. This results improves the understanding of the reaction mechanisms of various electrocatalytic reactions.
中文翻译:

原位拉曼光谱揭示了 Cu(111) 和多晶 Cu 表面的早期电化学氧化
研究在明确定义的 Cu 单晶衬底上吸附的中间物种的化学性质对于理解许多电催化反应至关重要。在此,我们使用原位壳隔离纳米粒子增强拉曼光谱 (SHINERS) 系统地研究了不同 pH 电解质中 Cu(111) 和多晶 Cu 表面电化学氧化的早期阶段。在 Cu(111) 上,我们首次发现了表面 OH 物质,它们在碱性 (0.01 M KOH) 和中性 (0.1 M Na2SO4) 电解质中形成 Cu2O 之前转化为化学吸附的“O”;而在 Cu(poly) 表面,我们只检测到表面氢氧化物的存在。而在强酸性溶液 (0.1 M H2SO4) 中,硫酸盐会取代羟基/氧基物质。
更新日期:2019-07-21
中文翻译:

原位拉曼光谱揭示了 Cu(111) 和多晶 Cu 表面的早期电化学氧化
研究在明确定义的 Cu 单晶衬底上吸附的中间物种的化学性质对于理解许多电催化反应至关重要。在此,我们使用原位壳隔离纳米粒子增强拉曼光谱 (SHINERS) 系统地研究了不同 pH 电解质中 Cu(111) 和多晶 Cu 表面电化学氧化的早期阶段。在 Cu(111) 上,我们首次发现了表面 OH 物质,它们在碱性 (0.01 M KOH) 和中性 (0.1 M Na2SO4) 电解质中形成 Cu2O 之前转化为化学吸附的“O”;而在 Cu(poly) 表面,我们只检测到表面氢氧化物的存在。而在强酸性溶液 (0.1 M H2SO4) 中,硫酸盐会取代羟基/氧基物质。































 京公网安备 11010802027423号
京公网安备 11010802027423号