当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, pharmacological and structural studies of 5-substituted-3-(1-arylmethyl-1,2,3,6-tetrahydropyridin-4-yl)-1H-indoles as multi-target ligands of aminergic GPCRs.
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-07-20 , DOI: 10.1016/j.ejmech.2019.07.050 Magda Kondej 1 , Tomasz M Wróbel 2 , Andrea G Silva 3 , Piotr Stępnicki 1 , Oliwia Koszła 1 , Ewa Kędzierska 4 , Agata Bartyzel 5 , Grażyna Biała 4 , Dariusz Matosiuk 1 , Maria I Loza 3 , Marián Castro 3 , Agnieszka A Kaczor 6
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-07-20 , DOI: 10.1016/j.ejmech.2019.07.050 Magda Kondej 1 , Tomasz M Wróbel 2 , Andrea G Silva 3 , Piotr Stępnicki 1 , Oliwia Koszła 1 , Ewa Kędzierska 4 , Agata Bartyzel 5 , Grażyna Biała 4 , Dariusz Matosiuk 1 , Maria I Loza 3 , Marián Castro 3 , Agnieszka A Kaczor 6
Affiliation
|
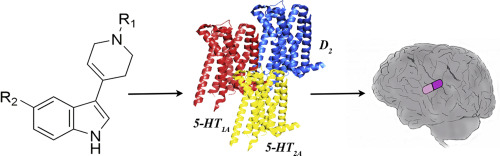
Schizophrenia is a complex disease with not fully understood pathomechanism, involving many neurotransmitters and their receptors. This is why it is best treated with multi-target drugs, such as second generation antipsychotics. Here we present 5-substituted-3-(1-arylmethyl-1,2,3,6-tetrahydropyridin-4-yl)-1H-indoles (1-20) which are ligands of dopamine D2 and serotonin 5-HT1A and 5-HT2A receptors and display affinity in the nanomolar range. These compounds were designed as modifications of the virtual hit experimentally confirmed, D2AAK1, and synthesized from indole or 5-alkoxyindoles and N-substituted piperidin-4-ones in methanol in the presence of potassium hydroxide. Compound 9 was subjected to X-ray studies and it crystallizes in the centrosymmetric monoclinic space group P21/c with one molecule in an asymmetric unit. Three most potent compounds (5, 9 and 17) turned out to be antagonists of both D2 and 5-HT2A receptors what is beneficial for their potential application as antipsychotics. Compound 5 was subjected to behavioral studies and exhibited antipsychotic, pro-cognitive and antidepressant activity in appropriate mice models. Structure-activity relationships for compounds 1-20 were rationalized using molecular docking. It was found that, in general, bulky C5-alkoxy substituents at the indole moiety are not favorable as they direct towards aqueous environment of the extracellular vestibule. Keywords: antipsychotics; behavioral studies, G protein-coupled receptors; indole derivatives; multi-target compounds; schizophrenia.
中文翻译:

5取代的3-(1-芳甲基-1,2,3,6-四氢吡啶-4--4-基)-1H-吲哚作为胺能GPCR的多靶点配体的合成,药理和结构研究。
精神分裂症是一种复杂的疾病,其发病机理尚未完全明了,涉及许多神经递质及其受体。这就是为什么最好用多靶点药物(例如第二代抗精神病药)对其进行治疗的原因。在这里,我们介绍了多巴胺D2和5-羟色胺5-HT1A和5的配体5-取代-3-(1-芳甲基-1,2,3,6-四氢吡啶-4--4-基)-1H-吲哚(1-20) -HT2A受体在纳摩尔范围内显示亲和力。这些化合物被设计为经实验证实的虚拟命中化合物D2AAK1的修饰,并在甲醇中,在氢氧化钾的存在下,由吲哚或5-烷氧基吲哚和N-取代的哌啶-4-酮合成。对化合物9进行了X射线研究,它在不对称单元中有一个分子的中心对称单斜空间群P21 / c中结晶。三种最有效的化合物(5、9和17)被证明是D2和5-HT2A受体的拮抗剂,这对于它们作为抗精神病药的潜在应用是有益的。对化合物5进行了行为研究,并在适当的小鼠模型中显示出抗精神病药,促认知药和抗抑郁药的活性。使用分子对接使化合物1-20的结构活性关系合理化。已经发现,通常,在吲哚部分的大体积C5-烷氧基取代基是不利的,因为它们直接指向细胞外前庭的水性环境。关键词:抗精神病药;抗精神病药 行为研究,G蛋白偶联受体;吲哚衍生物 多目标化合物 精神分裂症。9和17)既是D2受体又是5-HT2A受体的拮抗剂,这对于它们作为抗精神病药的潜在应用是有益的。对化合物5进行了行为研究,并在适当的小鼠模型中显示出抗精神病药,促认知药和抗抑郁药的活性。使用分子对接使化合物1-20的结构活性关系合理化。已经发现,通常,在吲哚部分的大体积C5-烷氧基取代基是不利的,因为它们直接指向细胞外前庭的水性环境。关键词:抗精神病药;抗精神病药 行为研究,G蛋白偶联受体;吲哚衍生物 多目标化合物 精神分裂症。9和17)既是D2受体又是5-HT2A受体的拮抗剂,这对于它们作为抗精神病药的潜在应用是有益的。对化合物5进行了行为研究,并在适当的小鼠模型中显示出抗精神病药,促认知药和抗抑郁药的活性。使用分子对接使化合物1-20的结构活性关系合理化。已经发现,通常,在吲哚部分的大体积C5-烷氧基取代基是不利的,因为它们直接指向细胞外前庭的水性环境。关键词:抗精神病药;抗精神病药 行为研究,G蛋白偶联受体;吲哚衍生物 多目标化合物 精神分裂症。使用分子对接使化合物1-20的结构活性关系合理化。已经发现,通常,在吲哚部分的大体积C5-烷氧基取代基是不利的,因为它们直接指向细胞外前庭的水性环境。关键词:抗精神病药;抗精神病药 行为研究,G蛋白偶联受体;吲哚衍生物 多目标化合物 精神分裂症。使用分子对接使化合物1-20的结构活性关系合理化。已经发现,通常,在吲哚部分的大体积C5-烷氧基取代基是不利的,因为它们直接指向细胞外前庭的水性环境。关键词:抗精神病药;抗精神病药 行为研究,G蛋白偶联受体;吲哚衍生物 多目标化合物 精神分裂症。
更新日期:2019-07-20
中文翻译:

5取代的3-(1-芳甲基-1,2,3,6-四氢吡啶-4--4-基)-1H-吲哚作为胺能GPCR的多靶点配体的合成,药理和结构研究。
精神分裂症是一种复杂的疾病,其发病机理尚未完全明了,涉及许多神经递质及其受体。这就是为什么最好用多靶点药物(例如第二代抗精神病药)对其进行治疗的原因。在这里,我们介绍了多巴胺D2和5-羟色胺5-HT1A和5的配体5-取代-3-(1-芳甲基-1,2,3,6-四氢吡啶-4--4-基)-1H-吲哚(1-20) -HT2A受体在纳摩尔范围内显示亲和力。这些化合物被设计为经实验证实的虚拟命中化合物D2AAK1的修饰,并在甲醇中,在氢氧化钾的存在下,由吲哚或5-烷氧基吲哚和N-取代的哌啶-4-酮合成。对化合物9进行了X射线研究,它在不对称单元中有一个分子的中心对称单斜空间群P21 / c中结晶。三种最有效的化合物(5、9和17)被证明是D2和5-HT2A受体的拮抗剂,这对于它们作为抗精神病药的潜在应用是有益的。对化合物5进行了行为研究,并在适当的小鼠模型中显示出抗精神病药,促认知药和抗抑郁药的活性。使用分子对接使化合物1-20的结构活性关系合理化。已经发现,通常,在吲哚部分的大体积C5-烷氧基取代基是不利的,因为它们直接指向细胞外前庭的水性环境。关键词:抗精神病药;抗精神病药 行为研究,G蛋白偶联受体;吲哚衍生物 多目标化合物 精神分裂症。9和17)既是D2受体又是5-HT2A受体的拮抗剂,这对于它们作为抗精神病药的潜在应用是有益的。对化合物5进行了行为研究,并在适当的小鼠模型中显示出抗精神病药,促认知药和抗抑郁药的活性。使用分子对接使化合物1-20的结构活性关系合理化。已经发现,通常,在吲哚部分的大体积C5-烷氧基取代基是不利的,因为它们直接指向细胞外前庭的水性环境。关键词:抗精神病药;抗精神病药 行为研究,G蛋白偶联受体;吲哚衍生物 多目标化合物 精神分裂症。9和17)既是D2受体又是5-HT2A受体的拮抗剂,这对于它们作为抗精神病药的潜在应用是有益的。对化合物5进行了行为研究,并在适当的小鼠模型中显示出抗精神病药,促认知药和抗抑郁药的活性。使用分子对接使化合物1-20的结构活性关系合理化。已经发现,通常,在吲哚部分的大体积C5-烷氧基取代基是不利的,因为它们直接指向细胞外前庭的水性环境。关键词:抗精神病药;抗精神病药 行为研究,G蛋白偶联受体;吲哚衍生物 多目标化合物 精神分裂症。使用分子对接使化合物1-20的结构活性关系合理化。已经发现,通常,在吲哚部分的大体积C5-烷氧基取代基是不利的,因为它们直接指向细胞外前庭的水性环境。关键词:抗精神病药;抗精神病药 行为研究,G蛋白偶联受体;吲哚衍生物 多目标化合物 精神分裂症。使用分子对接使化合物1-20的结构活性关系合理化。已经发现,通常,在吲哚部分的大体积C5-烷氧基取代基是不利的,因为它们直接指向细胞外前庭的水性环境。关键词:抗精神病药;抗精神病药 行为研究,G蛋白偶联受体;吲哚衍生物 多目标化合物 精神分裂症。






























 京公网安备 11010802027423号
京公网安备 11010802027423号