Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2019-07-20 , DOI: 10.1016/j.bioorg.2019.103129 Zhongbin Cheng 1 , Yuanli Li 2 , Wei Xu 3 , Wan Liu 2 , Lijun Liu 2 , Daigui Zhu 2 , Ying Kang 4 , Zhuhua Luo 3 , Qin Li 1
|
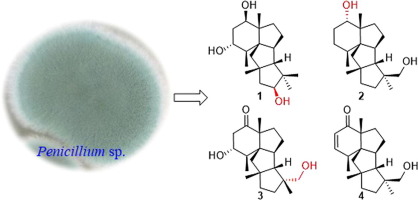
Cyclopianes, featuring a highly rigid 6/5/5/5-fused tetracyclic framework, are structurally unique and biologically significant and belong to a rarely reported diterpenoid family. Chemical investigation of an EtOAc extract of a deep-sea-derived Penicillium sp. led to the isolation of three new cyclopiane diterpenes, namely, conidiogenols C–D (1–2) and conidiogenone L (3). The structures were determined by extensive analyses of the spectroscopic data in association with ECD calculations and chemical conversion for configurational assignments. Compound 1 represents the second example of cyclopianes bearing a hydroxyl group at C-13. Compound 2, the third example of conidiogenols, possesses a distinct α-oriented 1-hydroxy group relative to other analogues. The bioassay study demonstrated that compounds 2 and 4–6 exhibited moderate inhibitory effects against five esophageal cancer cell lines with IC50 values ranging from 25 to 55 μM. The cytotoxicities of all compounds toward esophageal cancer cell lines were evaluated for the first time.
中文翻译:

来自深海真菌Penicillium sp。的三种新的环庚烷型二萜。YPGA11及其对人食道癌细胞的作用。
Cyclopianes具有高度刚性的6/5/5/5融合四环骨架,具有独特的结构和生物学意义,属于罕见的二萜类化合物。深海衍生的青霉菌种的EtOAc提取物的化学研究。导致了三个新cyclopiane双萜的分离,即,conidiogenols C-d(1 - 2)和conidiogenone L(3)。结构是通过对光谱数据进行广泛分析,结合ECD计算和化学转化进行结构确定的。化合物1代表在C-13处带有羟基的环戊烷的第二个实例。化合物2,香豆酚的第三个例子,相对于其他类似物,具有不同的α-定向的1-羟基。生物测定研究表明,化合物2和4 - 6表现出对与IC 5个食道癌细胞系中度抑制效果50个值范围从25到55微米。首次评估了所有化合物对食道癌细胞系的细胞毒性。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号