当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Monovalent and Divalent Cations at the α-Al2O3(0001)/Water Interface: How Cation Identity Affects Interfacial Ordering and Vibrational Dynamics
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-07-19 , DOI: 10.1021/acs.jpcc.9b01618
Stefan M. Piontek 1 , Aashish Tuladhar 1, 2 , Tim Marshall 1 , Eric Borguet 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-07-19 , DOI: 10.1021/acs.jpcc.9b01618
Stefan M. Piontek 1 , Aashish Tuladhar 1, 2 , Tim Marshall 1 , Eric Borguet 1
Affiliation
|
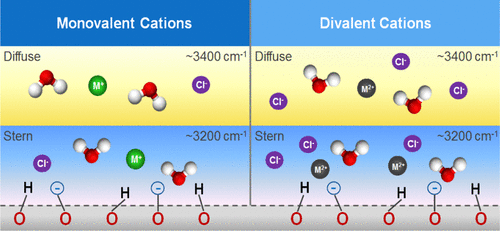
Mineral oxide/water interfaces are important for a wide range of industrial, geochemical, and biological processes. The reactivity of these interfaces is strongly impacted by the presence of ions. Thus, it is critical to understand how ions alter the interfacial environment. This can be achieved by measuring the changes in the structure and vibrational dynamics of interfacial water induced by the presence of ions in close vicinity to the mineral surface. The α-Al2O3(0001) surface represents a flexible platform to study the effect of ions on interfacial aqueous environments at positive, neutral, and negative surface charges. By using vibrational sum frequency generation (vSFG) in the frequency and time domains, we investigate how monovalent and divalent cations affect the hydrogen bonding environment of the first few layers of interfacial water next to α-Al2O3(0001). Our results indicate that monovalent cations, such as Li+, Na+, K+, and Cs+, appear to have lower binding affinities at the interface compared to Ca2+, Sr2+, and Ba2+. This leads to an interfacial region that is structured in a cation valence dependent manner. The addition of divalent cations at the negatively charged interface (pH 10) increases the spectral intensity in the 3400 cm–1 region compared to neat pH 10 H2O, in contrast to monovalent cations that only attenuate the vSFG signal. Time-resolved vSFG measurements reveal that the O–H vibrational lifetime (T1) of interfacial species at pH 10 in the presence of NaCl and BaCl2 remains similar. The restructuring of the interface seen in steady-state vSFG is manifested in the degree to which strongly hydrogen-bonded species recover to their original populations post excitation. By tracking the accumulation of ions at the interface via the vSFG response, we can characterize the unique surface arrangements of interfacial water molecules induced by monovalent and divalent cations at the α-Al2O3(0001)/water interface.
中文翻译:

α-Al2O3(0001)/水界面上的一价和二价阳离子:阳离子身份如何影响界面有序性和振动动力学
矿物氧化物/水的界面对于广泛的工业,地球化学和生物过程很重要。这些界面的反应性受到离子的存在的强烈影响。因此,了解离子如何改变界面环境至关重要。这可以通过测量由于紧邻矿物表面存在离子而引起的界面水的结构和振动动力学变化来实现。所述α-Al 2 ö 3(0001)表面代表一种柔性平台,用于研究离子在正,中性和负表面电荷对界面水环境的影响。通过在频域和时域中使用振动和频产生(vSFG),我们研究的一价和二价阳离子是如何影响的界面水最初几层的氢键环境旁边的α-Al 2 ö 3(0001)。我们的结果表明,与Ca 2 +,Sr 2+和Ba 2+相比,一价阳离子(例如Li +,Na +,K +和Cs +)在界面处的结合亲和力更低。。这导致以阳离子价依赖性方式构造的界面区域。与仅使vSFG信号衰减的单价阳离子相比,与纯pH 10 H 2 O相比,在带负电荷的界面(pH 10)上添加二价阳离子会增加3400 cm –1区域的光谱强度。时间分辨的vSFG测量表明,在存在NaCl和BaCl 2的情况下,在pH 10时界面物种的OH振动寿命(T 1)仍然相似。稳态vSFG中观察到的界面重组表现为强烈氢键结合的物质在激发后恢复到其原始种群的程度。通过跟踪离子在经由vSFG响应的界面处的积累,我们可以表征在所述α-Al诱导的一价和二价阳离子的界面的水分子的独特的表面安排2 ö 3(0001)/水界面。
更新日期:2019-07-19
中文翻译:

α-Al2O3(0001)/水界面上的一价和二价阳离子:阳离子身份如何影响界面有序性和振动动力学
矿物氧化物/水的界面对于广泛的工业,地球化学和生物过程很重要。这些界面的反应性受到离子的存在的强烈影响。因此,了解离子如何改变界面环境至关重要。这可以通过测量由于紧邻矿物表面存在离子而引起的界面水的结构和振动动力学变化来实现。所述α-Al 2 ö 3(0001)表面代表一种柔性平台,用于研究离子在正,中性和负表面电荷对界面水环境的影响。通过在频域和时域中使用振动和频产生(vSFG),我们研究的一价和二价阳离子是如何影响的界面水最初几层的氢键环境旁边的α-Al 2 ö 3(0001)。我们的结果表明,与Ca 2 +,Sr 2+和Ba 2+相比,一价阳离子(例如Li +,Na +,K +和Cs +)在界面处的结合亲和力更低。。这导致以阳离子价依赖性方式构造的界面区域。与仅使vSFG信号衰减的单价阳离子相比,与纯pH 10 H 2 O相比,在带负电荷的界面(pH 10)上添加二价阳离子会增加3400 cm –1区域的光谱强度。时间分辨的vSFG测量表明,在存在NaCl和BaCl 2的情况下,在pH 10时界面物种的OH振动寿命(T 1)仍然相似。稳态vSFG中观察到的界面重组表现为强烈氢键结合的物质在激发后恢复到其原始种群的程度。通过跟踪离子在经由vSFG响应的界面处的积累,我们可以表征在所述α-Al诱导的一价和二价阳离子的界面的水分子的独特的表面安排2 ö 3(0001)/水界面。

































 京公网安备 11010802027423号
京公网安备 11010802027423号