当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural basis of tubulin detyrosination by VASH2/SVBP heterodimer.
Nature Communications ( IF 14.7 ) Pub Date : 2019-07-19 , DOI: 10.1038/s41467-019-11277-8 Chen Zhou 1 , Ling Yan 1 , Wen-Hui Zhang 1 , Zhu Liu 1
Nature Communications ( IF 14.7 ) Pub Date : 2019-07-19 , DOI: 10.1038/s41467-019-11277-8 Chen Zhou 1 , Ling Yan 1 , Wen-Hui Zhang 1 , Zhu Liu 1
Affiliation
|
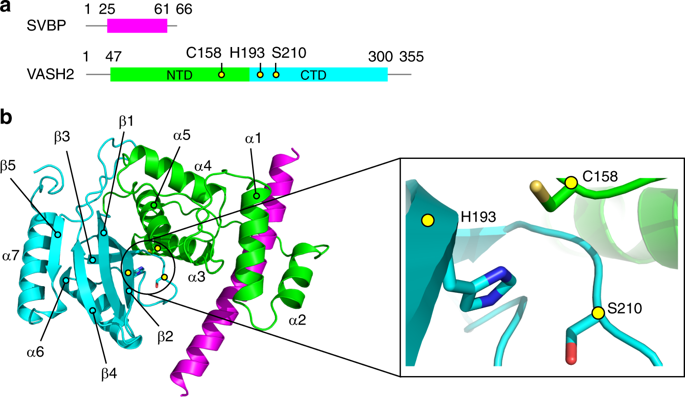
The C-terminus of α-tubulin undergoes a detyrosination/tyrosination cycle and dysregulation of this cycle is associated with cancer and other diseases. The molecular mechanisms of tubulin tyrosination are well studied, however it has remained unknown how tyrosine is cleaved from the tubulin tail. Here, we report the crystal structure of the long-sought detyrosination enzyme, the VASH2/SVBP heterodimer at 2.2 Å resolution and the structure of the tail/VASH2/SVBP complex at 2.5 Å resolution. VASH2 possesses a non-canonical Cys-His-Ser catalytic architecture for tyrosine cleavage. The dynamics of the α1- and α2- helices of VASH2 are related to the insolubility of VASH2. SVBP plays a chaperone-like role by extensively interacting with VASH2 and stabilizing these dynamic helices. A positively charged groove around the catalytic pocket and the α1- and α2- helices of VASH2 targets the tubulin tail for detyrosination. We provide insights into the mechanisms underlying the cycle of tubulin tyrosine cleavage and religation.
中文翻译:

VASH2 / SVBP异二聚体对微管蛋白脱酪氨酸的结构基础。
α-微管蛋白的C末端经历了脱酪氨酸/酪氨酸循环,该循环的失调与癌症和其他疾病有关。微管蛋白酪氨酸化的分子机制已得到很好的研究,但是如何从微管蛋白尾巴上裂解酪氨酸仍是未知的。在这里,我们报告了长期寻求的脱酪氨酸酶的晶体结构,分辨率为2.2Å的VASH2 / SVBP异二聚体和分辨率为2.5Å的尾巴/ VASH2 / SVBP复合物的结构。VASH2具有非经典的Cys-His-Ser催化结构,可用于酪氨酸裂解。VASH2的α1-和α2-螺旋的动力学与VASH2的不溶性有关。SVBP通过与VASH2广泛相互作用并稳定这些动态螺旋而起到类似伴侣的作用。围绕催化袋的正电荷槽以及VASH2的α1-和α2-螺旋靶向微管蛋白尾巴进行脱酪氨酸作用。我们提供了微管蛋白酪氨酸裂解和重新连接的循环机制的见解。
更新日期:2019-07-19
中文翻译:

VASH2 / SVBP异二聚体对微管蛋白脱酪氨酸的结构基础。
α-微管蛋白的C末端经历了脱酪氨酸/酪氨酸循环,该循环的失调与癌症和其他疾病有关。微管蛋白酪氨酸化的分子机制已得到很好的研究,但是如何从微管蛋白尾巴上裂解酪氨酸仍是未知的。在这里,我们报告了长期寻求的脱酪氨酸酶的晶体结构,分辨率为2.2Å的VASH2 / SVBP异二聚体和分辨率为2.5Å的尾巴/ VASH2 / SVBP复合物的结构。VASH2具有非经典的Cys-His-Ser催化结构,可用于酪氨酸裂解。VASH2的α1-和α2-螺旋的动力学与VASH2的不溶性有关。SVBP通过与VASH2广泛相互作用并稳定这些动态螺旋而起到类似伴侣的作用。围绕催化袋的正电荷槽以及VASH2的α1-和α2-螺旋靶向微管蛋白尾巴进行脱酪氨酸作用。我们提供了微管蛋白酪氨酸裂解和重新连接的循环机制的见解。





























 京公网安备 11010802027423号
京公网安备 11010802027423号