当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
PIDA‐Promoted Selective C5 C−H Selenylations of Indolines via Weak Interactions
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2019-09-12 , DOI: 10.1002/adsc.201900766 Linghui Gu 1 , Xinyue Fang 1 , Zhengyun Weng 1 , Jiafu Lin 1 , Meicui He 1 , Wenbo Ma 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2019-09-12 , DOI: 10.1002/adsc.201900766 Linghui Gu 1 , Xinyue Fang 1 , Zhengyun Weng 1 , Jiafu Lin 1 , Meicui He 1 , Wenbo Ma 1
Affiliation
|
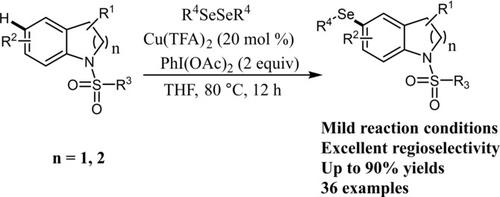
An efficient PIDA (phenyliodine(III) diacetate)‐promoted positional selective C−H selenylations of indolines with diaryl diselenides has been developed. This transformation conducted under mild reaction conditions with a broad functional group tolerance, thus providing an efficient protocol to selenylated indolines. Preliminary mechanistic studies indicated a SET pathway was likely involved in this selenylation reaction.
中文翻译:

PIDA通过弱相互作用促进二氢吲哚的选择性C 5 C H H硒基化
已经开发出一种有效的PIDA(二乙酸苯基碘(III))促进二芳基二硒化物与二氢吲哚的位置选择性CH硒基化反应。该转化在温和的反应条件下进行,具有宽泛的官能团耐受性,因此提供了硒化二氢吲哚的有效方法。初步的机理研究表明,SET途径可能参与了该硒化反应。
更新日期:2019-09-12
中文翻译:

PIDA通过弱相互作用促进二氢吲哚的选择性C 5 C H H硒基化
已经开发出一种有效的PIDA(二乙酸苯基碘(III))促进二芳基二硒化物与二氢吲哚的位置选择性CH硒基化反应。该转化在温和的反应条件下进行,具有宽泛的官能团耐受性,因此提供了硒化二氢吲哚的有效方法。初步的机理研究表明,SET途径可能参与了该硒化反应。































 京公网安备 11010802027423号
京公网安备 11010802027423号