Catalysis Today ( IF 5.2 ) Pub Date : 2018-02-24 , DOI: 10.1016/j.cattod.2018.02.046 Petr Novotný , Seif Yusuf , Fanxing Li , H. Henry Lamb
|
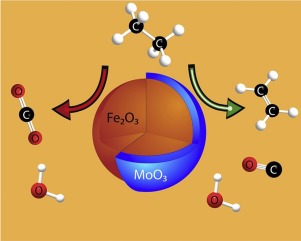
Oxidative dehydrogenation (ODH) of ethane offers large reductions in energy consumption and associated greenhouse gas emissions when compared to conventional steam cracking for ethylene production; however, catalytic ODH of ethane using co-fed O2 requires expensive air separation. As an alternative, we are investigating novel core-shell catalysts that utilize lattice oxygen (O2−) as the sole oxidant and operate in a cyclic redox mode. In this work, redox catalysts having 1, 3 and 6 monolayer (ML) equivalents of MoO3 on α-Fe2O3 and a stoichiometric ferric molybdate, Fe2(MoO4)3, were prepared, characterized by powder x-ray diffraction (XRD), x-ray photoelectron spectroscopy (XPS), diffuse-reflectance infrared Fourier transform spectroscopy (DRIFTS), and temperature-programmed reduction (TPR) and evaluated for ethane ODH in a cyclic redox mode at 600 °C. The characterization data are consistent with a core-shell structure for the calcined MoO3/Fe2O3 catalysts with a mixed Mo-Fe oxide surface layer. H2 and ethane TPR evidence that the shell inhibits Fe2O3 reduction and decreases the ethane combustion activity of the fully oxidized catalyst. Covering the Fe2O3 core with MoO3 also increases ODH activity and ethylene selectivity. In cyclic redox mode at 600 °C, ethylene selectivity was 57–62% for catalysts with 3 and 6 ML equivalents of MoO3.
中文翻译:

使用MoO 3 / Fe 2 O 3催化剂以循环氧化还原模式对乙烷进行氧化脱氢
与用于乙烯生产的常规蒸汽裂解法相比,乙烷的氧化脱氢(ODH)可大幅降低能耗和相关的温室气体排放。然而,使用共进料的O 2催化乙烷的ODH需要昂贵的空气分离。作为替代方案,我们正在研究利用晶格氧(O 2-)作为唯一氧化剂并以循环氧化还原模式运行的新型核-壳型催化剂。在这项工作中,具有的MoO的1,3和6的单层(ML)当量氧化还原催化剂3上的α-Fe 2 ö 3和化学计量钼酸铁,铁2(的MoO 4)3制备后,通过粉末X射线衍射(XRD),X射线光电子能谱(XPS),漫反射红外傅里叶变换光谱(DRIFTS)和程序升温还原(TPR)对其进行了表征,并评估了乙醇中的ODH含量。 600°C时的循环氧化还原模式。表征数据与具有混合的Mo-Fe氧化物表面层的煅烧的MoO 3 / Fe 2 O 3催化剂的核-壳结构一致。H 2和乙烷TPR证明壳抑制了Fe 2 O 3的还原并降低了完全氧化催化剂的乙烷燃烧活性。用MoO 3覆盖Fe 2 O 3芯还增加了ODH活性和乙烯的选择性。在600°C的循环氧化还原模式下,对于3和6 ML当量的MoO 3,催化剂的乙烯选择性为57-62%。































 京公网安备 11010802027423号
京公网安备 11010802027423号