当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Trapping and Characterization of Nontoxic Aβ42 Aggregation Intermediates.
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2019-08-01 , DOI: 10.1021/acschemneuro.9b00340 Alejandro R Foley 1 , Thomas S Finn 1 , Timothy Kung 1 , Asa Hatami 2 , Hsiau-Wei Lee 1 , Manping Jia 3 , Marco Rolandi 3 , Jevgenij A Raskatov 1
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2019-08-01 , DOI: 10.1021/acschemneuro.9b00340 Alejandro R Foley 1 , Thomas S Finn 1 , Timothy Kung 1 , Asa Hatami 2 , Hsiau-Wei Lee 1 , Manping Jia 3 , Marco Rolandi 3 , Jevgenij A Raskatov 1
Affiliation
|
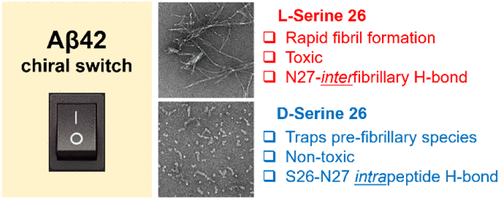
Amyloid β (Aβ) 42 is an aggregation-prone peptide and the believed seminal etiological agent of Alzheimer's disease (AD). Intermediates of Aβ42 aggregation, commonly referred to as diffusible oligomers, are considered to be among the most toxic forms of the peptide. Here, we studied the effect of the age-related epimerization of Ser26 (i.e., S26s chiral edit) in Aβ42 and discovered that this subtle molecular change led to reduced fibril formation propensity. Surprisingly, the resultant soluble aggregates were nontoxic. To gain insight into the structural changes that occurred in the peptide upon S26s substitution, the system was probed using an array of biophysical and biochemical methods. These experiments consistently pointed to the stabilization of aggregation intermediates in the Aβ42-S26s system. To better understand the changes arising as a consequence of the S26s substitution, molecular level structural studies were performed. Using a combined nuclear magnetic resonance (NMR)- and density functional theory (DFT)-computational approach, we found that the S26s chiral edit induced only local structural changes in the Gly25-Ser26-Asn27 region. Interestingly, these subtle changes enabled the formation of an intramolecular Ser26-Asn27 H-bond, which disrupted the ability of Asn27 to engage in the fibrillogenic side chain-to-side chain H-bonding pattern. This reveals that intermolecular stabilizing interactions between Asn27 side chains are a key element controlling Aβ42 aggregation and toxicity.
中文翻译:

无毒Aβ42聚集中间体的捕集和表征。
淀粉样蛋白β(Aβ)42是易于聚集的肽,是阿尔茨海默氏病(AD)的公认的病因。Aβ42聚集的中间体(通常称为可扩散的寡聚物)被认为是该肽最有毒的形式之一。在这里,我们研究了Aβ42中Ser26的年龄相关差向异构化(即S26的手性编辑)的影响,并发现这种细微的分子变化导致原纤维形成倾向降低。令人惊讶地,所得的可溶性聚集体是无毒的。为了深入了解S26s取代后肽中发生的结构变化,使用了一系列生物物理和生化方法对系统进行了探测。这些实验一致地指出了Aβ42-S26s系统中聚集中间体的稳定。为了更好地理解由于S26s取代而引起的变化,我们进行了分子水平的结构研究。使用组合的核磁共振(NMR)-和密度泛函理论(DFT)-计算方法,我们发现S26s手性编辑仅诱导Gly25-Ser26-Asn27区域的局部结构变化。有趣的是,这些细微的变化使分子内Ser26-Asn27 H键的形成成为可能,这破坏了Asn27参与原纤维形成的侧链至侧链H键结构的能力。这表明Asn27侧链之间的分子间稳定相互作用是控制Aβ42聚集和毒性的关键因素。使用组合的核磁共振(NMR)-和密度泛函理论(DFT)-计算方法,我们发现S26s手性编辑仅诱导Gly25-Ser26-Asn27区域的局部结构变化。有趣的是,这些细微的变化使分子内Ser26-Asn27 H键的形成成为可能,这破坏了Asn27参与原纤维形成的侧链至侧链H键结构的能力。这表明Asn27侧链之间的分子间稳定相互作用是控制Aβ42聚集和毒性的关键因素。使用组合的核磁共振(NMR)-和密度泛函理论(DFT)-计算方法,我们发现S26s手性编辑仅诱导Gly25-Ser26-Asn27区域的局部结构变化。有趣的是,这些细微的变化使分子内Ser26-Asn27 H键的形成成为可能,这破坏了Asn27参与原纤维形成的侧链至侧链H键结构的能力。这表明Asn27侧链之间的分子间稳定相互作用是控制Aβ42聚集和毒性的关键因素。这破坏了Asn27参与原纤维形成的侧链至侧链H键结构的能力。这表明Asn27侧链之间的分子间稳定相互作用是控制Aβ42聚集和毒性的关键因素。这破坏了Asn27参与原纤维形成的侧链至侧链H键结构的能力。这表明Asn27侧链之间的分子间稳定相互作用是控制Aβ42聚集和毒性的关键因素。
更新日期:2019-07-18
中文翻译:

无毒Aβ42聚集中间体的捕集和表征。
淀粉样蛋白β(Aβ)42是易于聚集的肽,是阿尔茨海默氏病(AD)的公认的病因。Aβ42聚集的中间体(通常称为可扩散的寡聚物)被认为是该肽最有毒的形式之一。在这里,我们研究了Aβ42中Ser26的年龄相关差向异构化(即S26的手性编辑)的影响,并发现这种细微的分子变化导致原纤维形成倾向降低。令人惊讶地,所得的可溶性聚集体是无毒的。为了深入了解S26s取代后肽中发生的结构变化,使用了一系列生物物理和生化方法对系统进行了探测。这些实验一致地指出了Aβ42-S26s系统中聚集中间体的稳定。为了更好地理解由于S26s取代而引起的变化,我们进行了分子水平的结构研究。使用组合的核磁共振(NMR)-和密度泛函理论(DFT)-计算方法,我们发现S26s手性编辑仅诱导Gly25-Ser26-Asn27区域的局部结构变化。有趣的是,这些细微的变化使分子内Ser26-Asn27 H键的形成成为可能,这破坏了Asn27参与原纤维形成的侧链至侧链H键结构的能力。这表明Asn27侧链之间的分子间稳定相互作用是控制Aβ42聚集和毒性的关键因素。使用组合的核磁共振(NMR)-和密度泛函理论(DFT)-计算方法,我们发现S26s手性编辑仅诱导Gly25-Ser26-Asn27区域的局部结构变化。有趣的是,这些细微的变化使分子内Ser26-Asn27 H键的形成成为可能,这破坏了Asn27参与原纤维形成的侧链至侧链H键结构的能力。这表明Asn27侧链之间的分子间稳定相互作用是控制Aβ42聚集和毒性的关键因素。使用组合的核磁共振(NMR)-和密度泛函理论(DFT)-计算方法,我们发现S26s手性编辑仅诱导Gly25-Ser26-Asn27区域的局部结构变化。有趣的是,这些细微的变化使分子内Ser26-Asn27 H键的形成成为可能,这破坏了Asn27参与原纤维形成的侧链至侧链H键结构的能力。这表明Asn27侧链之间的分子间稳定相互作用是控制Aβ42聚集和毒性的关键因素。这破坏了Asn27参与原纤维形成的侧链至侧链H键结构的能力。这表明Asn27侧链之间的分子间稳定相互作用是控制Aβ42聚集和毒性的关键因素。这破坏了Asn27参与原纤维形成的侧链至侧链H键结构的能力。这表明Asn27侧链之间的分子间稳定相互作用是控制Aβ42聚集和毒性的关键因素。















































 京公网安备 11010802027423号
京公网安备 11010802027423号