当前位置:
X-MOL 学术
›
Pest Manag. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hybrid-type strigolactone analogues derived from auxins.
Pest Management Science ( IF 3.8 ) Pub Date : 2019-08-23 , DOI: 10.1002/ps.5553 Daniel Blanco-Ania 1 , Jurgen J Mateman 1 , Adéla Hýlová 2 , Lukáš Spíchal 2 , Luc M Debie 1 , Binne Zwanenburg 1
Pest Management Science ( IF 3.8 ) Pub Date : 2019-08-23 , DOI: 10.1002/ps.5553 Daniel Blanco-Ania 1 , Jurgen J Mateman 1 , Adéla Hýlová 2 , Lukáš Spíchal 2 , Luc M Debie 1 , Binne Zwanenburg 1
Affiliation
|
BACKGROUND
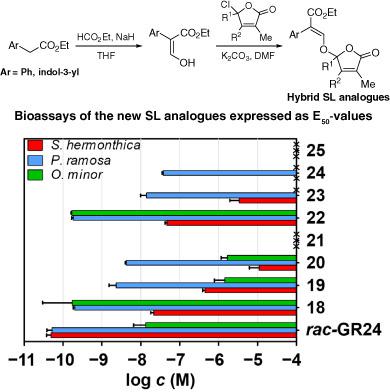
Strigolactones (SLs) have a vast number of ecological implications because of the broad spectrum of their biological activities. Unfortunately, the limited availability of SLs restricts their applicability for the benefit of humanity and renders synthesis the only option for their production. However, the structural complexity of SLs impedes their economical synthesis, which is unfeasible on a large scale. Synthesis of SL analogues and mimics with a simpler structure, but with retention of bioactivity, is the solution to this problem.
RESULTS
Here, we present eight new hybrid-type SL analogues derived from auxin, synthesized via coupling of auxin ester [ethyl 2-(1H-indol-3-yl)acetate] and of ethyl 2-phenylacetate with four D-rings (mono-, two di- and trimethylated). The new hybrid-type SL analogues were bioassayed to assess the germination activity of seeds of the parasitic weeds Striga hermonthica, Orobanche minor and Phelipanche ramosa using the classical method of counting germinated seeds and a colorimetric method. The bioassays revealed that analogues with a natural monomethylated D-ring had appreciable to good activity towards the three species and were the most active derivatives. By contrast, derivatives with the trimethylated D-ring showed no activity. The dimethylated derivatives (2,4-dimethyl and 3,4-dimethyl) were slightly active, especially towards P. ramosa.
CONCLUSIONS
New hybrid-type analogues derived from auxins have been prepared. These analogues may be attractive as potential suicidal germination agents for parasitic weed control because of their ease of preparation and relevant bioactivity. © 2019 The Authors. Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
中文翻译:

来源于生长素的杂合型strigolactone类似物。
背景技术Strigolactones(SLs)由于其生物活性的广谱性而具有大量的生态意义。不幸的是,SL的有限可用性限制了它们对人类有益的适用性,并使合成成为其生产的唯一选择。但是,SL的结构复杂性阻碍了它们的经济合成,这在大规模上是不可行的。解决此问题的方法是合成具有更简单结构但具有生物活性的SL类似物和模拟物。结果在这里,我们介绍了八种新的生长素衍生的混合型SL类似物,它们是通过将生长素酯[2-(1H-吲哚-3-基)乙酸乙酯]和2-苯基乙酸乙酯与四个D环偶联而成的。 -,两个二甲基和三甲基化)。使用计数发芽种子的经典方法和比色法,对新的杂交型SL类似物进行了生物测定,以评估寄生杂草Striga hermonthica,Orobanche minor和Phelipanche ramosa种子的萌发活性。生物测定表明,具有天然单甲基化D环的类似物对这三个物种具有明显的良好活性,并且是活性最高的衍生物。相反,具有三甲基化D环的衍生物没有活性。二甲基化衍生物(2,4-二甲基和3,4-二甲基)具有轻微的活性,特别是对P. ramosa。结论已经制备了从生长素衍生的新的杂合型类似物。这些类似物由于其易于制备和相关的生物活性而作为潜在的自杀性发芽剂用于寄生杂草控制可能是有吸引力的。©2019作者。约翰·威利父子有限公司(John Wiley&Sons Ltd)代表化学工业协会出版的《害虫管理科学》。
更新日期:2019-08-23
中文翻译:

来源于生长素的杂合型strigolactone类似物。
背景技术Strigolactones(SLs)由于其生物活性的广谱性而具有大量的生态意义。不幸的是,SL的有限可用性限制了它们对人类有益的适用性,并使合成成为其生产的唯一选择。但是,SL的结构复杂性阻碍了它们的经济合成,这在大规模上是不可行的。解决此问题的方法是合成具有更简单结构但具有生物活性的SL类似物和模拟物。结果在这里,我们介绍了八种新的生长素衍生的混合型SL类似物,它们是通过将生长素酯[2-(1H-吲哚-3-基)乙酸乙酯]和2-苯基乙酸乙酯与四个D环偶联而成的。 -,两个二甲基和三甲基化)。使用计数发芽种子的经典方法和比色法,对新的杂交型SL类似物进行了生物测定,以评估寄生杂草Striga hermonthica,Orobanche minor和Phelipanche ramosa种子的萌发活性。生物测定表明,具有天然单甲基化D环的类似物对这三个物种具有明显的良好活性,并且是活性最高的衍生物。相反,具有三甲基化D环的衍生物没有活性。二甲基化衍生物(2,4-二甲基和3,4-二甲基)具有轻微的活性,特别是对P. ramosa。结论已经制备了从生长素衍生的新的杂合型类似物。这些类似物由于其易于制备和相关的生物活性而作为潜在的自杀性发芽剂用于寄生杂草控制可能是有吸引力的。©2019作者。约翰·威利父子有限公司(John Wiley&Sons Ltd)代表化学工业协会出版的《害虫管理科学》。















































 京公网安备 11010802027423号
京公网安备 11010802027423号