Nature Communications ( IF 14.7 ) Pub Date : 2019-07-18 , DOI: 10.1038/s41467-019-11134-8 Nuligonda Thirupathi 1 , Fang Wei 1 , Chen-Ho Tung 1 , Zhenghu Xu 1, 2
|
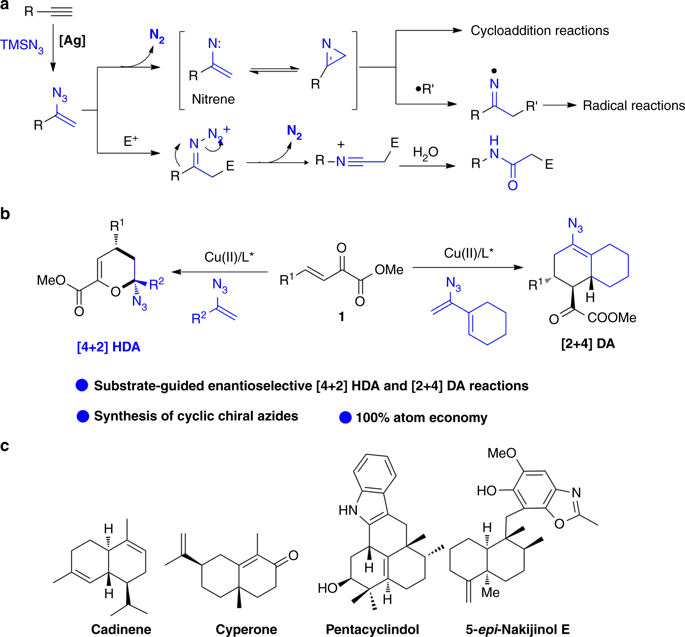
Vinyl azides, bearing conjugated azide and alkene functional groups, have been recognized as versatile building blocks in organic synthesis. In general vinyl azides act as 3-atom (CCN) synthons through the fast release of molecular nitrogen and have been extensively utilized in the construction of structurally diverse N-heterocycles. Keeping the azide moiety intact in organic transformations to synthesis chiral azides is an important but challenging task. Herein, we report an enantioselective copper(II)/BOX-catalyzed cycloaddition of vinyl azides, generating diverse chiral cyclic azides. α-Aryl substituted vinyl azides react with unsaturated keto esters through an inverse-electron-demand hetero-Diels-Alder reaction to afford chiral azido dihydropyrans with excellent enatioselectivities. In contrast, cyclohexenyl azides undergo a diastereo- and enantio-selective Diels-Alder reaction giving important azido octahydronaphthalenes with three continuous stereogenic centers. Notable features of these reactions include a very broad scope, mild reaction conditions and 100% atom economy.
中文翻译:

通过乙烯基叠氮化物的不对称环加成反应发散合成手性环状叠氮化物。
带有共轭叠氮化物和烯烃官能团的乙烯基叠氮化物已被公认为有机合成中的通用结构单元。通常,叠氮化乙烯基通过分子氮的快速释放而充当3-原子(CCN)合成子,并已广泛用于构建结构多样的N-杂环。在有机转化为合成手性叠氮化物的过程中保持叠氮化物部分完整是一项重要但具有挑战性的任务。本文中,我们报道了乙烯基叠氮化物的对映选择性铜(II)/ BOX催化的环加成反应,生成了多种手性环状叠氮化物。α-芳基取代的乙烯基叠氮化物通过反电子需求的杂Diels-Alder反应与不饱和酮酯反应,得到具有出色对映选择性的手性叠氮基二氢吡喃。相反,环己烯基叠氮化物进行非对映和对映选择性的Diels-Alder反应,得到具有三个连续立体中心的重要叠氮八氢萘。这些反应的显着特征包括范围很广,反应条件温和和100%的原子经济性。































 京公网安备 11010802027423号
京公网安备 11010802027423号