当前位置:
X-MOL 学术
›
Cell Death Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Shifting the balance of autophagy and proteasome activation reduces proteotoxic cell death: a novel therapeutic approach for restoring photoreceptor homeostasis.
Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-07-18 , DOI: 10.1038/s41419-019-1780-1 Yaoyan Qiu 1, 2 , Jingyu Yao 1 , Lin Jia 1 , Debra A Thompson 1, 3 , David N Zacks 1
Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-07-18 , DOI: 10.1038/s41419-019-1780-1 Yaoyan Qiu 1, 2 , Jingyu Yao 1 , Lin Jia 1 , Debra A Thompson 1, 3 , David N Zacks 1
Affiliation
|
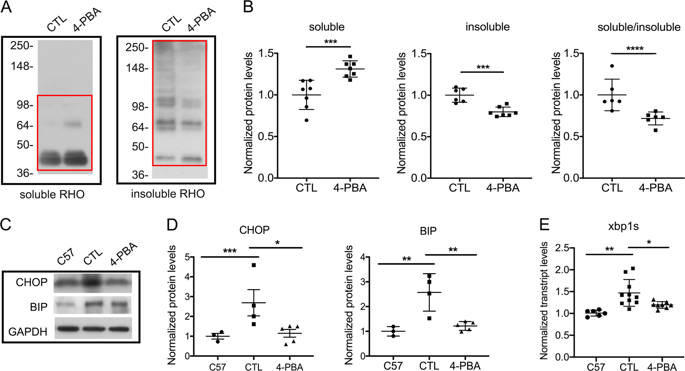
The P23H variant of rhodopsin results in misfolding of the protein, and is a common cause of the blinding disease autosomal dominant retinitis pigmentosa (adRP). We have recently demonstrated that degeneration of photoreceptor cells in retinas of P23H mice is due to the endoplasmic reticulum stress (ERS)-induced activation of autophagy that leads to a secondary proteasome insufficiency and activation of cell death pathways. We propose that this increased level of autophagy flux relative to proteasome activity, which we term the A:P ratio, represents a marker of altered photoreceptor cell homeostasis, and that therapies aimed at normalizing this ratio will result in increased photoreceptor cell survival. To test this postulate, we treated P23H mice with a chemical chaperone (4-phenylbutyric acid) to improve rhodopsin folding, or with a selective phosphodiesterase-4 inhibitor (rolipram) to increase proteasome activity. P23H mice treated with either of these agents exhibited reduced ERS, decreased autophagy flux, increased proteasome activity, and decreased activation of cell death pathways. In addition, rates of retinal degeneration were decreased, and photoreceptor morphology and visual function were preserved. These findings support the conclusion that normalizing the A:P ratio, either by reducing the ERS-induced activation of autophagy, or by increasing proteasome activity, improves photoreceptor survival, and suggest a potential new therapeutic strategy for the treatment of adRP caused by protein folding defects.
中文翻译:

改变自噬和蛋白酶体活化的平衡可减少蛋白毒性细胞死亡:恢复光感受器稳态的一种新的治疗方法。
视紫红质的P23H变体导致蛋白质错误折叠,并且是致盲性疾病常染色体显性遗传性视网膜色素变性(adRP)的常见原因。我们最近已经证明,P23H小鼠视网膜中感光细胞的变性是由于内质网应激(ERS)诱导的自噬激活,导致继发的蛋白酶体功能不全和细胞死亡途径的激活。我们提出相对于蛋白酶体活性的这种自噬通量的增加水平(我们称为A:P比值)代表了改变的感光细胞稳态的标志,并且旨在使该比例正常化的疗法将导致感光细胞存活率的提高。为了验证这一假设,我们用化学分子伴侣(4-苯基丁酸)对P23H小鼠进行了处理,以改善视紫红质的折叠能力,或与选择性磷酸二酯酶4抑制剂(咯利普兰)一起使用以增加蛋白酶体的活性。用这些试剂中的任何一种处理的P23H小鼠均表现出ERS降低,自噬通量降低,蛋白酶体活性提高和细胞死亡途径激活降低。另外,降低了视网膜变性的速率,并保留了感光体形态和视觉功能。这些发现支持这样的结论,即通过降低ERS诱导的自噬激活或增加蛋白酶体活性来使A:P比值正常化,可以改善感光细胞的存活率,并提出了潜在的新治疗策略,用于治疗由蛋白质折叠引起的adRP缺陷。自噬通量减少,蛋白酶体活性增加和细胞死亡途径的激活减少。另外,降低了视网膜变性的速率,并保留了感光体形态和视觉功能。这些发现支持这样的结论,即通过降低ERS诱导的自噬激活或增加蛋白酶体活性来使A:P比值正常化,可以改善光感受器的存活率,并提出了潜在的新治疗策略,用于治疗由蛋白质折叠引起的adRP缺陷。自噬通量减少,蛋白酶体活性增加和细胞死亡途径的激活减少。另外,降低了视网膜变性的速率,并保留了感光体形态和视觉功能。这些发现支持这样的结论,即通过降低ERS诱导的自噬激活或增加蛋白酶体活性来使A:P比值正常化,可以改善光感受器的存活率,并提出了潜在的新治疗策略,用于治疗由蛋白质折叠引起的adRP缺陷。
更新日期:2019-07-18
中文翻译:

改变自噬和蛋白酶体活化的平衡可减少蛋白毒性细胞死亡:恢复光感受器稳态的一种新的治疗方法。
视紫红质的P23H变体导致蛋白质错误折叠,并且是致盲性疾病常染色体显性遗传性视网膜色素变性(adRP)的常见原因。我们最近已经证明,P23H小鼠视网膜中感光细胞的变性是由于内质网应激(ERS)诱导的自噬激活,导致继发的蛋白酶体功能不全和细胞死亡途径的激活。我们提出相对于蛋白酶体活性的这种自噬通量的增加水平(我们称为A:P比值)代表了改变的感光细胞稳态的标志,并且旨在使该比例正常化的疗法将导致感光细胞存活率的提高。为了验证这一假设,我们用化学分子伴侣(4-苯基丁酸)对P23H小鼠进行了处理,以改善视紫红质的折叠能力,或与选择性磷酸二酯酶4抑制剂(咯利普兰)一起使用以增加蛋白酶体的活性。用这些试剂中的任何一种处理的P23H小鼠均表现出ERS降低,自噬通量降低,蛋白酶体活性提高和细胞死亡途径激活降低。另外,降低了视网膜变性的速率,并保留了感光体形态和视觉功能。这些发现支持这样的结论,即通过降低ERS诱导的自噬激活或增加蛋白酶体活性来使A:P比值正常化,可以改善感光细胞的存活率,并提出了潜在的新治疗策略,用于治疗由蛋白质折叠引起的adRP缺陷。自噬通量减少,蛋白酶体活性增加和细胞死亡途径的激活减少。另外,降低了视网膜变性的速率,并保留了感光体形态和视觉功能。这些发现支持这样的结论,即通过降低ERS诱导的自噬激活或增加蛋白酶体活性来使A:P比值正常化,可以改善光感受器的存活率,并提出了潜在的新治疗策略,用于治疗由蛋白质折叠引起的adRP缺陷。自噬通量减少,蛋白酶体活性增加和细胞死亡途径的激活减少。另外,降低了视网膜变性的速率,并保留了感光体形态和视觉功能。这些发现支持这样的结论,即通过降低ERS诱导的自噬激活或增加蛋白酶体活性来使A:P比值正常化,可以改善光感受器的存活率,并提出了潜在的新治疗策略,用于治疗由蛋白质折叠引起的adRP缺陷。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号