Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy
Science ( IF 44.7 ) Pub Date : 2015-11-05 , DOI: 10.1126/science.aac4255 Ayelet Sivan 1 , Leticia Corrales 1 , Nathaniel Hubert 2 , Jason B. Williams 1 , Keston Aquino-Michaels 3 , Zachary M. Earley 2 , Franco W. Benyamin 1 , Yuk Man Lei 2 , Bana Jabri 2 , Maria-Luisa Alegre 2 , Eugene B. Chang 2 , Thomas F. Gajewski 1, 2
Science ( IF 44.7 ) Pub Date : 2015-11-05 , DOI: 10.1126/science.aac4255 Ayelet Sivan 1 , Leticia Corrales 1 , Nathaniel Hubert 2 , Jason B. Williams 1 , Keston Aquino-Michaels 3 , Zachary M. Earley 2 , Franco W. Benyamin 1 , Yuk Man Lei 2 , Bana Jabri 2 , Maria-Luisa Alegre 2 , Eugene B. Chang 2 , Thomas F. Gajewski 1, 2
Affiliation
|
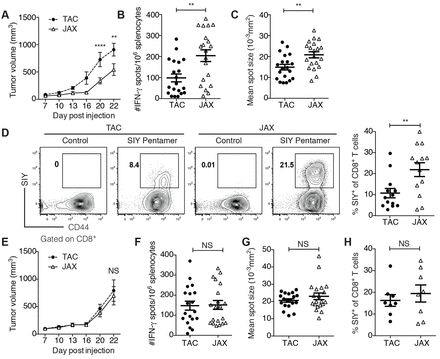
Gut microbes affect immunotherapy The unleashing of antitumor T cell responses has ushered in a new era of cancer treatment. Although these therapies can cause dramatic tumor regressions in some patients, many patients inexplicably see no benefit. Mice have been used in two studies to investigate what might be happening. Specific members of the gut microbiota influence the efficacy of this type of immunotherapy (see the Perspective by Snyder et al.). Vétizou et al. found that optimal responses to anticytotoxic T lymphocyte antigen blockade required specific Bacteroides spp. Similarly, Sivan et al. discovered that Bifidobacterium spp. enhanced the efficacy of antiprogrammed cell death ligand 1 therapy. Science, this issue, p. 1079 and p. 1084; see also p. 1031 Gut microbes modulate the effectiveness of cancer immunotherapies in mice. [Also see Perspective by Snyder et al.] T cell infiltration of solid tumors is associated with favorable patient outcomes, yet the mechanisms underlying variable immune responses between individuals are not well understood. One possible modulator could be the intestinal microbiota. We compared melanoma growth in mice harboring distinct commensal microbiota and observed differences in spontaneous antitumor immunity, which were eliminated upon cohousing or after fecal transfer. Sequencing of the 16S ribosomal RNA identified Bifidobacterium as associated with the antitumor effects. Oral administration of Bifidobacterium alone improved tumor control to the same degree as programmed cell death protein 1 ligand 1 (PD-L1)–specific antibody therapy (checkpoint blockade), and combination treatment nearly abolished tumor outgrowth. Augmented dendritic cell function leading to enhanced CD8+ T cell priming and accumulation in the tumor microenvironment mediated the effect. Our data suggest that manipulating the microbiota may modulate cancer immunotherapy.
中文翻译:

共生双歧杆菌促进抗肿瘤免疫并促进抗 PD-L1 功效
肠道微生物影响免疫治疗 抗肿瘤 T 细胞反应的释放开启了癌症治疗的新时代。尽管这些疗法可以在某些患者中引起显着的肿瘤消退,但许多患者莫名其妙地看不到任何益处。有两项研究使用小鼠来调查可能发生的情况。肠道微生物群的特定成员会影响此类免疫疗法的功效(参见 Snyder 等人的观点)。维蒂祖等人。发现对抗细胞毒性 T 淋巴细胞抗原阻断的最佳反应需要特定的拟杆菌属。同样,Sivan 等人。发现双歧杆菌属。增强了抗程序性细胞死亡配体 1 疗法的功效。科学,这个问题,p。1079 和第。1084; 另见第。1031 肠道微生物调节小鼠癌症免疫疗法的有效性。[另见 Snyder 等人的观点。] 实体瘤的 T 细胞浸润与良好的患者预后相关,但个体之间可变免疫反应的机制尚不清楚。一种可能的调节剂可能是肠道微生物群。我们比较了具有不同共生微生物群的小鼠的黑色素瘤生长,并观察到自发抗肿瘤免疫的差异,这些差异在同居或粪便转移后被消除。16S 核糖体 RNA 的测序鉴定出双歧杆菌与抗肿瘤作用相关。单独口服双歧杆菌改善肿瘤控制的程度与程序性细胞死亡蛋白 1 配体 1 (PD-L1) 特异性抗体治疗(检查点阻断)相同,联合治疗几乎消除了肿瘤的生长。增强的树突细胞功能导致增强的 CD8+ T 细胞启动和在肿瘤微环境中的积累介导了这种效应。我们的数据表明,操纵微生物群可能会调节癌症免疫治疗。
更新日期:2015-11-05
中文翻译:

共生双歧杆菌促进抗肿瘤免疫并促进抗 PD-L1 功效
肠道微生物影响免疫治疗 抗肿瘤 T 细胞反应的释放开启了癌症治疗的新时代。尽管这些疗法可以在某些患者中引起显着的肿瘤消退,但许多患者莫名其妙地看不到任何益处。有两项研究使用小鼠来调查可能发生的情况。肠道微生物群的特定成员会影响此类免疫疗法的功效(参见 Snyder 等人的观点)。维蒂祖等人。发现对抗细胞毒性 T 淋巴细胞抗原阻断的最佳反应需要特定的拟杆菌属。同样,Sivan 等人。发现双歧杆菌属。增强了抗程序性细胞死亡配体 1 疗法的功效。科学,这个问题,p。1079 和第。1084; 另见第。1031 肠道微生物调节小鼠癌症免疫疗法的有效性。[另见 Snyder 等人的观点。] 实体瘤的 T 细胞浸润与良好的患者预后相关,但个体之间可变免疫反应的机制尚不清楚。一种可能的调节剂可能是肠道微生物群。我们比较了具有不同共生微生物群的小鼠的黑色素瘤生长,并观察到自发抗肿瘤免疫的差异,这些差异在同居或粪便转移后被消除。16S 核糖体 RNA 的测序鉴定出双歧杆菌与抗肿瘤作用相关。单独口服双歧杆菌改善肿瘤控制的程度与程序性细胞死亡蛋白 1 配体 1 (PD-L1) 特异性抗体治疗(检查点阻断)相同,联合治疗几乎消除了肿瘤的生长。增强的树突细胞功能导致增强的 CD8+ T 细胞启动和在肿瘤微环境中的积累介导了这种效应。我们的数据表明,操纵微生物群可能会调节癌症免疫治疗。































 京公网安备 11010802027423号
京公网安备 11010802027423号