当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solar Irradiation Induced Transformation of Ferrihydrite in the Presence of Aqueous Fe2+
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2019-07-26 , DOI: 10.1021/acs.est.9b02750
Zhipeng Shu 1 , Lihu Liu 1 , Wenfeng Tan 1 , Steven L. Suib 2 , Guohong Qiu 1 , Xiong Yang 1 , Lirong Zheng 3 , Fan Liu 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2019-07-26 , DOI: 10.1021/acs.est.9b02750
Zhipeng Shu 1 , Lihu Liu 1 , Wenfeng Tan 1 , Steven L. Suib 2 , Guohong Qiu 1 , Xiong Yang 1 , Lirong Zheng 3 , Fan Liu 1
Affiliation
|
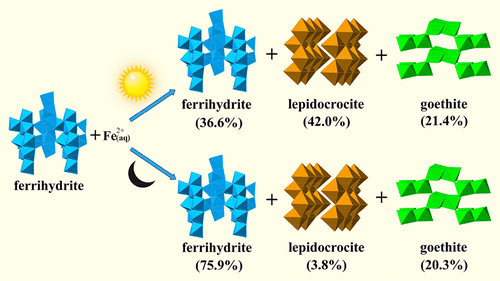
Ferrihydrite commonly occurs in soils and sediments, especially in acid mine drainage (AMD). Solar irradiation may affect Fe(II)-catalyzed transformation of metastable ferrihydrite to more stable iron oxides on AMD surface. We investigated the Fe(II)-catalyzed transformation process and mechanism of ferrihydrite under light irradiation. In nitrogen atmosphere, Fe2+aq could be oxidized to goethite and lepidocrocite by hydroxyl radical (OH•), superoxide radical (O2•–) and hole (hvb+) generated from ferrihydrite under ultraviolet (UV) irradiation (300–400 nm) at pH 6.0, and O2•– and hvb+ were mainly responsible for Fe2+aq oxidation. In addition, the ligand-to-metal charge-transfer (LMCT) process between Fe(II) and ferrihydrite could be promoted by UV irradiation. Goethite proportion increased with increasing Fe2+aq concentration. Both visible (vis) and solar irradiation could also lead to the oxidation of Fe2+aq to goethite and lepidocrocite, and the proportion of lepidocrocite increased with increasing light intensity. Fe2+aq was photochemically oxidized to schwertmannite at pH 3.0 and 4.5, and the oxidation rate was higher than that under dark conditions in air. The photochemical oxidation rate of Fe2+aq decreased in the presence of humic acid. This study facilitates a better understanding of the formation and transformation of iron oxides in natural environments and ancient Earth.
中文翻译:

Fe2 +存在下太阳辐射诱导水铁矿的转变
水铁矿通常存在于土壤和沉积物中,尤其是在酸性矿山排水(AMD)中。太阳辐射可能会影响Fe(II)催化的亚稳亚铁酸盐转变为AMD表面上更稳定的氧化铁。我们研究了Fe(II)催化光下水铁矿的转化过程和机理。在氮气氛中,Fe 2+水溶液可被紫外线(300-)照射下由亚铁酸盐生成的羟基自由基(OH •),超氧化物自由基(O 2 •-)和空穴(h vb +)氧化成针铁矿和纤铁矿。pH值为6.0时为400 nm),而O 2 •–和h vb +则主要是铁2+水溶液氧化。此外,紫外线辐射可以促进Fe(II)与水铁矿之间的配体到金属的电荷转移(LMCT)过程。针铁矿的比例随着Fe 2+水溶液浓度的增加而增加。可见光(可见光)和太阳辐射都可能导致Fe 2+ aq氧化为针铁矿和纤铁矿,并且纤铁矿的比例随着光强度的增加而增加。Fe 2+水溶液在pH 3.0和4.5下被光化学氧化为schwertmannite,其氧化速率高于在黑暗条件下的空气中。Fe 2+水溶液的光化学氧化速率在腐殖酸存在下降低。这项研究有助于更好地了解自然环境和古代地球中氧化铁的形成和转化。
更新日期:2019-07-26
中文翻译:

Fe2 +存在下太阳辐射诱导水铁矿的转变
水铁矿通常存在于土壤和沉积物中,尤其是在酸性矿山排水(AMD)中。太阳辐射可能会影响Fe(II)催化的亚稳亚铁酸盐转变为AMD表面上更稳定的氧化铁。我们研究了Fe(II)催化光下水铁矿的转化过程和机理。在氮气氛中,Fe 2+水溶液可被紫外线(300-)照射下由亚铁酸盐生成的羟基自由基(OH •),超氧化物自由基(O 2 •-)和空穴(h vb +)氧化成针铁矿和纤铁矿。pH值为6.0时为400 nm),而O 2 •–和h vb +则主要是铁2+水溶液氧化。此外,紫外线辐射可以促进Fe(II)与水铁矿之间的配体到金属的电荷转移(LMCT)过程。针铁矿的比例随着Fe 2+水溶液浓度的增加而增加。可见光(可见光)和太阳辐射都可能导致Fe 2+ aq氧化为针铁矿和纤铁矿,并且纤铁矿的比例随着光强度的增加而增加。Fe 2+水溶液在pH 3.0和4.5下被光化学氧化为schwertmannite,其氧化速率高于在黑暗条件下的空气中。Fe 2+水溶液的光化学氧化速率在腐殖酸存在下降低。这项研究有助于更好地了解自然环境和古代地球中氧化铁的形成和转化。































 京公网安备 11010802027423号
京公网安备 11010802027423号