当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
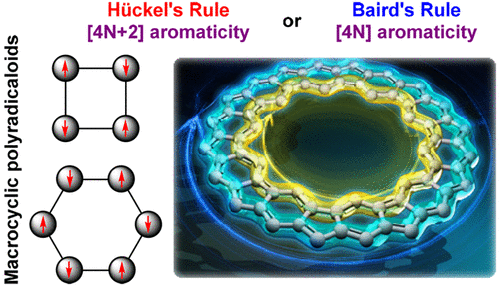
Global Aromaticity in Macrocyclic Polyradicaloids: Hückel’s Rule or Baird’s Rule?
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2019-07-17 00:00:00 , DOI: 10.1021/acs.accounts.9b00257 Chunchen Liu 1 , Yong Ni 1 , Xuefeng Lu 1 , Guangwu Li 1 , Jishan Wu 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2019-07-17 00:00:00 , DOI: 10.1021/acs.accounts.9b00257 Chunchen Liu 1 , Yong Ni 1 , Xuefeng Lu 1 , Guangwu Li 1 , Jishan Wu 1
Affiliation
|
Aromaticity is one of the most important concepts in organic chemistry to understand the electronic properties of cyclic π-conjugated molecules. Over a century, different aromaticity rules have been developed and validated. For planar monocyclic conjugated polyenes (also known as [n]annulenes), they will be aromatic if they contain [4N + 2] π electrons according to Hückel’s rule, or antiaromatic if they have [4N] π electrons. Topological change from a planar to a half-twisted Möbius strip will lead to [4N] ([4N + 2]) aromaticity (antiaromaticity), which is just inverse to Hückel’s rule. When the molecules are excited into the first triplet excited state, the Hückel (anti)aromaticity observed in the ground state will become reversed according to Baird’s rule. Strictly speaking, these basic rules are only applicable for monocyclic conjugated systems, but some polycyclic systems such as porphyrinoids may also follow these rules if there is a dominant [n]annulene-like conjugation pathway. On the other hand, all-benzenoid polycyclic aromatic hydrocarbons usually display local aromaticity with π electrons predominantly localized at certain benzene rings according to Clar’s aromatic sextet rule.
中文翻译:

大环多自由基的整体芳香性:Hückel法则还是Baird法则?
芳香性是有机化学中了解环状π共轭分子电子特性的最重要概念之一。一个多世纪以来,已经开发并验证了不同的芳香性规则。对于平面单环共轭多烯(也称为[ n ]环烯),如果它们根据Hückel规则包含[4 N + 2]π个电子,则它们将是芳族的;如果它们具有[4 N ]π个电子,则它们是抗芳族的。从平面扭曲到半扭曲的莫比乌斯带的拓扑变化将导致[4 N ]([4 N+ 2])芳香性(抗芳香性),与Hückel的定律恰好相反。当分子被激发到第一个三重激发态时,根据贝尔德定律,在基态下观察到的Hückel(反)芳香性将反转。严格来说,这些基本规则仅适用于单环共轭体系,但如果存在主要的[ n ]环戊烯样共轭途径,则某些多环体系(如卟啉类化合物)也可以遵循这些规则。另一方面,根据Clar的芳族六重体定律,全苯型多环芳烃通常表现出局部芳香性,其中π电子主要位于某些苯环上。
更新日期:2019-07-17
中文翻译:

大环多自由基的整体芳香性:Hückel法则还是Baird法则?
芳香性是有机化学中了解环状π共轭分子电子特性的最重要概念之一。一个多世纪以来,已经开发并验证了不同的芳香性规则。对于平面单环共轭多烯(也称为[ n ]环烯),如果它们根据Hückel规则包含[4 N + 2]π个电子,则它们将是芳族的;如果它们具有[4 N ]π个电子,则它们是抗芳族的。从平面扭曲到半扭曲的莫比乌斯带的拓扑变化将导致[4 N ]([4 N+ 2])芳香性(抗芳香性),与Hückel的定律恰好相反。当分子被激发到第一个三重激发态时,根据贝尔德定律,在基态下观察到的Hückel(反)芳香性将反转。严格来说,这些基本规则仅适用于单环共轭体系,但如果存在主要的[ n ]环戊烯样共轭途径,则某些多环体系(如卟啉类化合物)也可以遵循这些规则。另一方面,根据Clar的芳族六重体定律,全苯型多环芳烃通常表现出局部芳香性,其中π电子主要位于某些苯环上。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号