当前位置:
X-MOL 学术
›
Anal. Methods
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A more universal and stable method for lactate dehydrogenase isoenzyme test
Analytical Methods ( IF 2.7 ) Pub Date : 2019-07-17 00:00:00 , DOI: 10.1039/c9ay01327j Peijuan Li 1, 2, 3, 4 , Nanhai Qiu 3, 4, 5, 6 , Li Zhang 3, 4, 6, 7, 8 , Guishu Zhong 1, 2, 3, 4 , Fancai Zeng 3, 4, 6, 7, 8
Analytical Methods ( IF 2.7 ) Pub Date : 2019-07-17 00:00:00 , DOI: 10.1039/c9ay01327j Peijuan Li 1, 2, 3, 4 , Nanhai Qiu 3, 4, 5, 6 , Li Zhang 3, 4, 6, 7, 8 , Guishu Zhong 1, 2, 3, 4 , Fancai Zeng 3, 4, 6, 7, 8
Affiliation
|
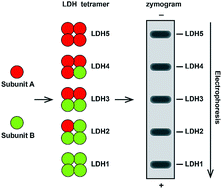
As an important enzyme for energy metabolism, lactate dehydrogenase (LDH) and its isoenzymes (LDH isoenzymes) have been extensively studied. LDH zymography is a common method to detect LDH isoenzymes, which offers the special advantage of directly observing 5 isozyme bands in the active state. But it has poor stability, and is difficult to apply for a variety of studies, so it has not been widely utilized. Here we have explored the influence of key factors including the temperature and pH of the running buffer, buffer type, and extraction methods on the detection of LDH isoforms and established a more practical and universal LDH zymography method, which can guarantee a stable and quality result, and be easily applied for different studies.
中文翻译:

乳酸脱氢酶同工酶检测的更通用,更稳定的方法
作为能量代谢的重要酶,乳酸脱氢酶(LDH)及其同工酶(LDH同工酶)已被广泛研究。LDH酶谱学是检测LDH同工酶的常用方法,它具有直接观察处于活动状态的5个同工酶条带的特殊优势。但是它的稳定性差,并且难以应用于各种研究中,因此尚未得到广泛的应用。在这里,我们探索了运行缓冲液的温度和pH,缓冲液类型和提取方法等关键因素对LDH亚型检测的影响,并建立了一种更实用,更通用的LDH酶法,可以确保结果的稳定和高质量。 ,并且可以轻松地应用于不同的研究。
更新日期:2019-07-17
中文翻译:

乳酸脱氢酶同工酶检测的更通用,更稳定的方法
作为能量代谢的重要酶,乳酸脱氢酶(LDH)及其同工酶(LDH同工酶)已被广泛研究。LDH酶谱学是检测LDH同工酶的常用方法,它具有直接观察处于活动状态的5个同工酶条带的特殊优势。但是它的稳定性差,并且难以应用于各种研究中,因此尚未得到广泛的应用。在这里,我们探索了运行缓冲液的温度和pH,缓冲液类型和提取方法等关键因素对LDH亚型检测的影响,并建立了一种更实用,更通用的LDH酶法,可以确保结果的稳定和高质量。 ,并且可以轻松地应用于不同的研究。































 京公网安备 11010802027423号
京公网安备 11010802027423号