Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2019-07-17 , DOI: 10.1016/j.jcis.2019.07.046
Zongchen Li , Xuemin Liu , Wei Jin , Qingsong Hu , Yaping Zhao
|
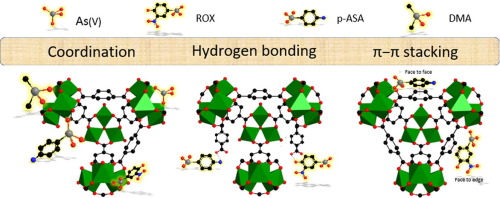
Arsenic species are regarded as typical water pollutants due to their toxicity. The chemical structures of arsenic species greatly influence their migration and transformation in the environment. Metal-organic frameworks (MOFs) are used as reliable adsorbents to control arsenic contamination, so it is urgently needed to study the effect of chemical structure of arsenic species during adsorption process. The adsorption behaviors of arsenate (As(V)) and its organic forms such as roxarsone (ROX), p-arsanilic acid (p-ASA) and dimethyl arsenate (DMA) by MIL-101(Fe), a type of highly porosity iron-based MOFs in aqueous environment were detailed investigated. The adsorption kinetics of those arsenic species on MIL-101(Fe) is rapid followed with pseudo-second-order kinetic model. MIL-101(Fe) exhibits excellent adsorption capacities for As(V), ROX, p-ASA and DMA with maximum adsorption capacities of 232.98, 507.97, 379.65 and 158.94 mg g−1, respectively. The formed FeO
As inner-sphere coordination between arsenic species and the incomplete-coordinated cationic Fe in the MIL-101(Fe) cluster is the primary adsorption mechanism based on FTIR and XPS analysis. Substituent aromatic units in ROX and p-ASA strengthen the adsorption on MIL-101(Fe) through hydrogen bonds and π-π stacking interaction, resulting in higher adsorption capacities far beyond that of As(V) and DMA. The reusability of MIL-101(Fe) is limited by the strong Fe
O
As coordination. These results confirm MIL-101(Fe) a reliable adsorbent to control the aqueous arsenic species contamination and emphasize the significant role of the chemical structure of arsenic speciation on adsorption performances of MOFs.
中文翻译:

砷在MIL-101(Fe)上的吸附行为:砷化学结构的作用
砷由于其毒性而被视为典型的水污染物。砷物种的化学结构极大地影响了它们在环境中的迁移和转化。金属有机骨架(MOFs)被用作控制砷污染的可靠吸附剂,因此迫切需要研究砷在吸附过程中化学结构的影响。一种高孔隙率的MIL-101(Fe)吸附砷酸盐(As(V))及其有机形式如罗沙酮(ROX),对砷酸(p-ASA)和砷酸二甲酯(DMA)的行为详细研究了水性环境中的铁基MOF。通过伪二级动力学模型快速跟踪了这些砷物质在MIL-101(Fe)上的吸附动力学。MIL-101(Fe)对As(V),ROX,-1。基于FTIR和XPS分析,MIL-101(Fe)团簇中砷物种与不完全配位的阳离子Fe之间形成的Fe O
As内部球配位是主要的吸附机理。ROX和p-ASA中的取代芳族单元通过氢键和π-π堆积相互作用增强了对MIL-101(Fe)的吸附,导致更高的吸附能力,远远超过了As(V)和DMA。MIL-101(Fe)的可重用性受到强的Fe
O
As配位作用的限制。这些结果证实了MIL-101(Fe)是一种可靠的吸附剂,可控制含水砷物质的污染,并强调了砷形态的化学结构对MOF吸附性能的重要作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号