Journal of Catalysis ( IF 6.5 ) Pub Date : 2019-07-17 , DOI: 10.1016/j.jcat.2019.06.051
Hanseul Choi , Sunyoung Oh , Si Bui Trung Tran , Jeong Young Park
|
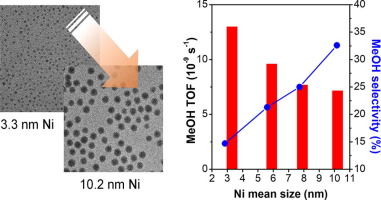
The effect of particle size for Ni nanoparticles supported on β-Ga2O3 was investigated for CO2 hydrogenation to methanol at 0.5 MPa. Model Ni nanoparticles ranging from 3.3 to 10.2 nm were synthesized using the hot injection method by controlling the reaction temperature and time. The smallest Ni nanoparticles (3.3 nm) showed the highest catalytic activity across the entire temperature range and the largest Ni nanoparticles (10.2 nm) showed the highest methanol selectivity. The apparent activation energies for methanol with Ni nanoparticles increased from 6.0 to 18.4 kcal mol−1 as the nanoparticle size increased. Furthermore, it was found that the smallest Ni nanoparticles favor the reverse water gas shift reaction. In situ DRIFT analysis revealed that the gallium oxide itself could produce an intermediate species and the addition of Ni on the oxide support increases the hydrogenation rate. The Ni supported catalysts showed a CO peak, but the smallest Ni nanoparticles showed a larger CO peak than that for the largest Ni nanoparticles, which clearly supports that the smaller nanoparticles favor the reverse water gas shift reaction.
中文翻译:

在Ga 2 O 3上进行尺寸控制的Ni型催化剂,用于CO 2加氢制甲醇
粒径对Ni的纳米颗粒负载于的β-Ga效果2 ö 3进行了研究用于CO 2在0.5MPa氢化成甲醇。通过控制反应温度和时间,使用热注射法合成了3.3至10.2 nm的模型Ni纳米颗粒。最小的Ni纳米颗粒(3.3 nm)在整个温度范围内显示出最高的催化活性,最大的Ni纳米颗粒(10.2 nm)显示出最高的甲醇选择性。带有Ni纳米粒子的甲醇的表观活化能从6.0增加到18.4 kcal mol -1随着纳米颗粒尺寸的增加。此外,发现最小的Ni纳米粒子有利于逆水煤气变换反应。原位DRIFT分析表明,氧化镓本身可以产生中间物种,并且在氧化物载体上添加Ni可以提高氢化速率。Ni负载的催化剂显示出CO峰,但是最小的Ni纳米颗粒显示出比最大的Ni纳米颗粒大的CO峰,这清楚地表明较小的纳米颗粒有利于反向水煤气变换反应。

































 京公网安备 11010802027423号
京公网安备 11010802027423号