Nature Communications ( IF 14.7 ) Pub Date : 2019-07-17 , DOI: 10.1038/s41467-019-11109-9
Zi-Lei Xia 1 , Chao Zheng 1 , Ren-Qi Xu 1 , Shu-Li You 1, 2
|
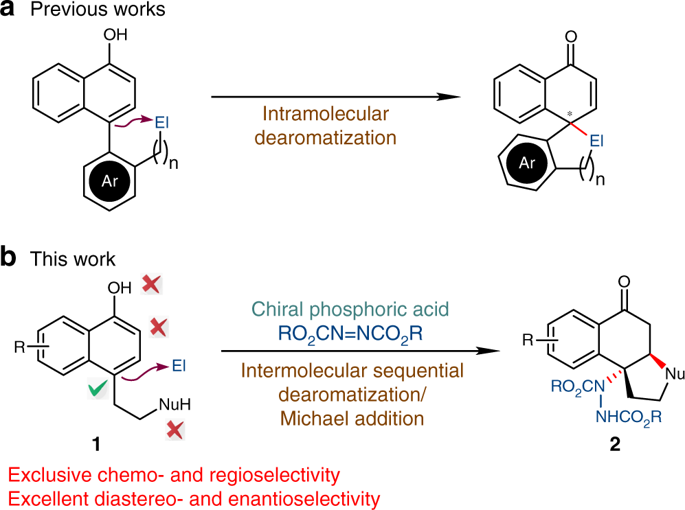
Asymmetric dearomatization reactions have recently emerged as a powerful tool for the rapid build-up of the molecular complexity. Chiral three-dimensional polycyclic molecules bearing contiguous stereogenic centers can be synthesized from readily available planar aromatic feedstocks. Here we report that an intermolecular asymmetric dearomatization reaction of α-naphthols bearing a tethered nucleophile at the C4 position of the naphthol ring is achieved by a chiral phosphoric acid. The reaction proceeds via a highly chemo- and regioselective aminative dearomatization/Michael addition sequence, affording a wide array of functionalized cyclic ketones in good yields (up to 93%) with excellent enantioselectivity (up to >99% ee). The catalyst loading can be reduced to 0.1 mol%. Preliminary mechanistic investigations identify that the enantioselectivity is established in the dearomatization step, while the Michael addition is the rate-limiting step. A working model accounting for the origin of the stereochemistry is proposed based on DFT calculations.
中文翻译:

手性磷酸催化α-萘酚/迈克尔加成序列的氨基脱芳香化作用。
最近,不对称脱芳香化反应已成为快速建立分子复杂性的有力工具。可以从容易获得的平面芳族原料合成带有连续立体中心的手性三维多环分子。在这里我们报道α的分子间不对称脱芳香化反应通过手性磷酸获得在萘酚环的C4位带有拴系亲核试剂的α-萘酚。反应通过高度的化学和区域选择性胺化脱芳香化反应/迈克尔加成序列进行,以良好的对映选择性(高达> 99%ee)以良好的收率(高达93%)提供了多种功能化的环状酮。催化剂的加入量可以降低到0.1mol%。初步的机械研究确定对映选择性是在脱芳香化步骤中建立的,而迈克尔加成是限速步骤。基于DFT计算,提出了解释立体化学起源的工作模型。







































 京公网安备 11010802027423号
京公网安备 11010802027423号