Synthesis ( IF 2.2 ) Pub Date : 2019-07-16 , DOI: 10.1055/s-0037-1610720 Santiago Fonzo 1 , Didier F. Vargas 1, 2 , Teodoro S. Kaufman 1, 2
|
Dedicated to the memory of our treasured colleague and Master Prof. Dr. Edmundo A. Rúveda (03/1934–12/2018) and our beloved friend and co-worker Lic. María Virginia Méndez (02/1987–01/2019).
Abstract
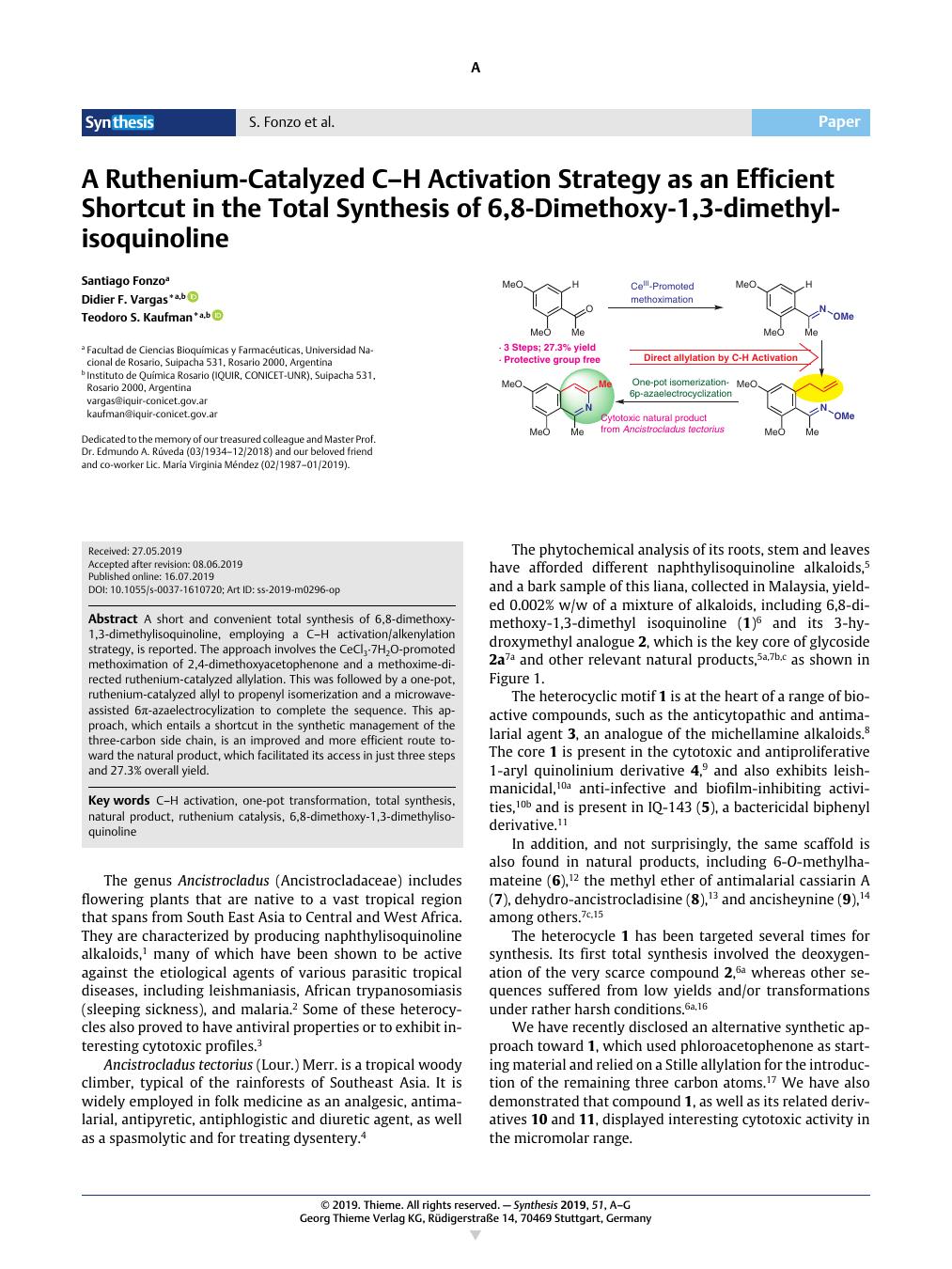
A short and convenient total synthesis of 6,8-dimethoxy-1,3-dimethylisoquinoline, employing a C–H activation/alkenylation strategy, is reported. The approach involves the CeCl3·7H2O-promoted methoximation of 2,4-dimethoxyacetophenone and a methoxime-directed ruthenium-catalyzed allylation. This was followed by a one-pot, ruthenium-catalyzed allyl to propenyl isomerization and a microwave-assisted 6π-azaelectrocylization to complete the sequence. This approach, which entails a shortcut in the synthetic management of the three-carbon side chain, is an improved and more efficient route toward the natural product, which facilitated its access in just three steps and 27.3% overall yield.
A short and convenient total synthesis of 6,8-dimethoxy-1,3-dimethylisoquinoline, employing a C–H activation/alkenylation strategy, is reported. The approach involves the CeCl3·7H2O-promoted methoximation of 2,4-dimethoxyacetophenone and a methoxime-directed ruthenium-catalyzed allylation. This was followed by a one-pot, ruthenium-catalyzed allyl to propenyl isomerization and a microwave-assisted 6π-azaelectrocylization to complete the sequence. This approach, which entails a shortcut in the synthetic management of the three-carbon side chain, is an improved and more efficient route toward the natural product, which facilitated its access in just three steps and 27.3% overall yield.
中文翻译:

钌催化的CH活化策略作为6,8-二甲氧基-1,3-二甲基异喹啉全合成中的有效捷径
谨记我们宝贵的同事和大师Edmundo A.Rúveda博士(03 / 1934–12 / 2018)以及我们挚爱的朋友和同事Lic。玛丽亚·弗吉尼亚·门德斯(02 / 1987–01 / 2019)。
抽象的
据报道,采用C–H活化/烯基化策略,可以方便,快捷地合成6,8-二甲氧基-1,3-二甲基异喹啉。该方法涉及由CeCl 3 ·7H 2 O促进的2,4-二甲氧基苯乙酮的甲氧基化和甲氧肟定向的钌催化的烯丙基化。随后是一锅钌催化的烯丙基到丙烯基的异构化反应,以及微波辅助的6π-氮杂电子化反应以完成序列。这种方法在三碳侧链的合成管理中需要捷径,是一种改进的,更有效的天然产物生产路线,只需三步即可获得,而且总收率达27.3%。
据报道,采用C–H活化/烯基化策略,可以方便,快捷地合成6,8-二甲氧基-1,3-二甲基异喹啉。该方法涉及由CeCl 3 ·7H 2 O促进的2,4-二甲氧基苯乙酮的甲氧基化和甲氧肟定向的钌催化的烯丙基化。随后是一锅钌催化的烯丙基到丙烯基的异构化反应,以及微波辅助的6π-氮杂电子化反应以完成序列。这种方法在三碳侧链的合成管理中需要捷径,是一种改进的,更有效的天然产物生产路线,只需三步即可获得,而且总收率达27.3%。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号