当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self-Developed Fabrication of Manganese Oxides Microtubes with Efficient Catalytic Performance for the Selective Oxidation of 5-Hydroxymethylfurfural
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2019-07-16 , DOI: 10.1021/acs.iecr.9b02650 Yuanjie Ma 1 , Ting Zhang 1 , Lifang Chen 1 , Hongye Cheng 1 , Zhiwen Qi 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2019-07-16 , DOI: 10.1021/acs.iecr.9b02650 Yuanjie Ma 1 , Ting Zhang 1 , Lifang Chen 1 , Hongye Cheng 1 , Zhiwen Qi 1
Affiliation
|
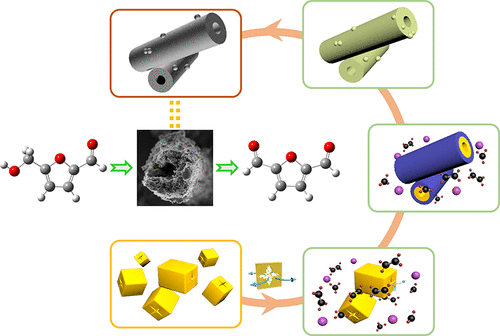
The development of green catalytic processes from biomass-derived feedstock such as 5-hydroxymethylfurfural (HMF) to valuable chemicals is a hot topic in which the facile synthesis of heterogeneous catalyst and molecular oxygen as the oxidant are desired. In this work, manganese oxide (MnOx) microtubes were prepared by a facile solvothermal method via in situ self-development, where chemical conversion coupled with physical Ostwald ripening occurred. The results showed that MnOx microtubes could be used as an efficient catalyst for the selective aerobic oxidation of HMF to 2,5-diformylfuran (DFF). Various reaction parameters have been investigated and DFF was obtained with a high selectivity of 100% under the reaction conditions of 90 °C, 8.0 bar O2, and N,N-dimethylformamide as solvent. Moreover, kinetic parameters revealed that the reaction fitted the first order equation corresponding to HMF. In particular, it was demonstrated that the activation energy was low with an 11.80 kcal/mol in comparison with reported non-noble catalysts, thus the reaction could be performed under a relatively low reaction temperature. Furthermore, the catalyst could be easily recoverable and reusable after several times without any significant loss, suggesting its excellent reusability and stability.
中文翻译:

自行开发的具有高效催化性能的5-羟基甲基糠醛选择性氧化锰氧化物微管
从生物质来源的原料(例如5-羟甲基糠醛(HMF))发展到有价值的化学品的绿色催化方法的发展是一个热门话题,其中需要容易地合成多相催化剂和分子氧作为氧化剂。在这项工作中,通过一种容易的溶剂热方法,通过原位自显影制备了氧化锰(MnO x)微管,其中发生了化学转化,并发生了物理奥斯特瓦尔德(Ostwald)成熟。结果表明,MnO x微管可用作将HMF选择性好氧氧化为2,5-二甲酰呋喃(DFF)的有效催化剂。已经研究了各种反应参数,并在90°C,8.0 bar O 2和90°C的反应条件下以100%的高选择性获得了DFF。N,N-二甲基甲酰胺作为溶剂。此外,动力学参数表明该反应符合对应于HMF的一阶方程。特别地,证明了与报道的非贵族催化剂相比,活化能低,为11.80kcal / mol,因此该反应可以在相对低的反应温度下进行。此外,该催化剂可以在数次之后容易地回收和再利用,而没有任何明显的损失,这表明它具有极好的可再利用性和稳定性。
更新日期:2019-07-17
中文翻译:

自行开发的具有高效催化性能的5-羟基甲基糠醛选择性氧化锰氧化物微管
从生物质来源的原料(例如5-羟甲基糠醛(HMF))发展到有价值的化学品的绿色催化方法的发展是一个热门话题,其中需要容易地合成多相催化剂和分子氧作为氧化剂。在这项工作中,通过一种容易的溶剂热方法,通过原位自显影制备了氧化锰(MnO x)微管,其中发生了化学转化,并发生了物理奥斯特瓦尔德(Ostwald)成熟。结果表明,MnO x微管可用作将HMF选择性好氧氧化为2,5-二甲酰呋喃(DFF)的有效催化剂。已经研究了各种反应参数,并在90°C,8.0 bar O 2和90°C的反应条件下以100%的高选择性获得了DFF。N,N-二甲基甲酰胺作为溶剂。此外,动力学参数表明该反应符合对应于HMF的一阶方程。特别地,证明了与报道的非贵族催化剂相比,活化能低,为11.80kcal / mol,因此该反应可以在相对低的反应温度下进行。此外,该催化剂可以在数次之后容易地回收和再利用,而没有任何明显的损失,这表明它具有极好的可再利用性和稳定性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号