当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solid-State Conversion of Scandium Phosphate into Scandium Oxide with Sodium Compounds
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2019-07-30 , DOI: 10.1021/acs.iecr.9b02411 Bengi Yagmurlu 1, 2 , Wenzhong Zhang 3 , Mikko J. Heikkilä 4 , Risto T. Koivula 3 , Bernd Friedrich 2
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2019-07-30 , DOI: 10.1021/acs.iecr.9b02411 Bengi Yagmurlu 1, 2 , Wenzhong Zhang 3 , Mikko J. Heikkilä 4 , Risto T. Koivula 3 , Bernd Friedrich 2
Affiliation
|
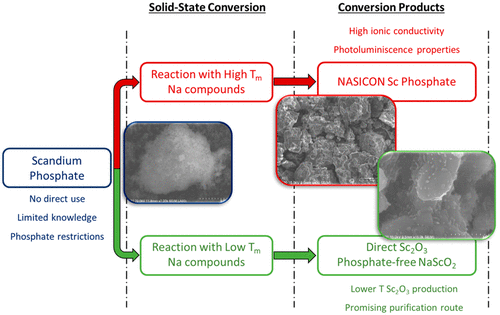
The application of scandium (Sc) is hindered by insufficient supply. The majority of the world Sc supply is sourced from industrial byproducts, where Sc needs to be separated from other components. Phosphate precipitation is an effective separation and purification method to harvest dissolved Sc ions from acidic leachate solutions; however the obtained Sc phosphate currently has no direct application. To this end, a solid-state conversion route of Sc phosphate to oxide was investigated by using five different sodium compounds, as sodium forms very stable phosphate compounds. The thermal conversion (up to 1000 °C) of Sc phosphate with high melting point sodium compounds (sodium sulfate, carbonate, and chloride) yielded a stable mixed sodium–scandium phosphate phase with a formula of Na3Sc2(PO4)3. The thermal conversion with lower melting point sodium compounds (sodium hydroxide and nitrate) resulted in the separation of Sc from phosphate moieties, forming respectively Sc oxides (NaScO2 or Sc2O3) and sodium phosphate. In situ high temperature X-ray diffraction, differential scanning calorimetry (DSC), and thermogravimetry (TGA) were employed to investigate the solid-state conversion process by sodium nitrate. Slower heating rate (120 °C/h) and the evolution of oxygen gas (as a result of sodium nitrate decomposition) favored the formation of Sc2O3 phase over NaScO2 phase, and the conversion reaction was completed at 670 °C. The conversion process was further explored as a purification step toward Sc-containing mixed phosphate precipitates, where the impurities (aluminum and iron phosphates) were converted into sodium aluminate and ferrite and could then be separated from Sc2O3 by their differences in acid/base solubility.
中文翻译:

用钠化合物将磷酸Scan固态转化为氧化Scan
supply(Sc)的应用由于供应不足而受到阻碍。世界上大部分Sc的供应都来自工业副产品,在工业副产品中,Sc需要与其他成分分开。磷酸盐沉淀是一种从酸性渗滤液溶液中收集溶解的Sc离子的有效分离和纯化方法。然而,目前所获得的磷酸Sc尚无直接应用。为此,通过使用五种不同的钠化合物研究了Sc磷酸盐向氧化物的固态转化途径,因为钠形成非常稳定的磷酸盐化合物。高熔点的钠化合物(硫酸钠,碳酸盐和氯化物)对磷酸Sc的热转化(高达1000°C)产生了稳定的磷酸钠–混合相,分子式为Na 3 Sc 2(PO 4)3。较低熔点的钠化合物(氢氧化钠和硝酸盐)的热转化导致Sc与磷酸盐部分分离,分别形成Sc氧化物(NaScO 2或Sc 2 O 3)和磷酸钠。利用原位高温X射线衍射,差示扫描量热法(DSC)和热重分析(TGA)研究了硝酸钠的固相转化过程。较慢的加热速率(120°C / h)和氧气的逸出(硝酸钠分解的结果)比NaScO 2有利于形成Sc 2 O 3相相,转化反应在670℃下完成。进一步探讨了将转化过程作为含Sc混合磷酸盐沉淀的纯化步骤,其中杂质(铝和磷酸铁)被转化为铝酸钠和铁素体,然后可以通过酸/酸的差异与Sc 2 O 3分离。碱溶解度。
更新日期:2019-07-30
中文翻译:

用钠化合物将磷酸Scan固态转化为氧化Scan
supply(Sc)的应用由于供应不足而受到阻碍。世界上大部分Sc的供应都来自工业副产品,在工业副产品中,Sc需要与其他成分分开。磷酸盐沉淀是一种从酸性渗滤液溶液中收集溶解的Sc离子的有效分离和纯化方法。然而,目前所获得的磷酸Sc尚无直接应用。为此,通过使用五种不同的钠化合物研究了Sc磷酸盐向氧化物的固态转化途径,因为钠形成非常稳定的磷酸盐化合物。高熔点的钠化合物(硫酸钠,碳酸盐和氯化物)对磷酸Sc的热转化(高达1000°C)产生了稳定的磷酸钠–混合相,分子式为Na 3 Sc 2(PO 4)3。较低熔点的钠化合物(氢氧化钠和硝酸盐)的热转化导致Sc与磷酸盐部分分离,分别形成Sc氧化物(NaScO 2或Sc 2 O 3)和磷酸钠。利用原位高温X射线衍射,差示扫描量热法(DSC)和热重分析(TGA)研究了硝酸钠的固相转化过程。较慢的加热速率(120°C / h)和氧气的逸出(硝酸钠分解的结果)比NaScO 2有利于形成Sc 2 O 3相相,转化反应在670℃下完成。进一步探讨了将转化过程作为含Sc混合磷酸盐沉淀的纯化步骤,其中杂质(铝和磷酸铁)被转化为铝酸钠和铁素体,然后可以通过酸/酸的差异与Sc 2 O 3分离。碱溶解度。

































 京公网安备 11010802027423号
京公网安备 11010802027423号