当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemo and regioselective lysine modification on native proteins
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-02-23 , DOI: 10.1021/jacs.7b12874 Maria J Matos 1 , Bruno L Oliveira 1 , Nuria Martínez-Sáez 1 , Ana Guerreiro 2 , Pedro M S D Cal 2 , Jean Bertoldo 1 , María Maneiro 3 , Elizabeth Perkins 4 , Julie Howard 5 , Michael J Deery 5 , Justin M Chalker 6 , Francisco Corzana 7 , Gonzalo Jiménez-Osés 7 , Gonçalo J L Bernardes 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-02-23 , DOI: 10.1021/jacs.7b12874 Maria J Matos 1 , Bruno L Oliveira 1 , Nuria Martínez-Sáez 1 , Ana Guerreiro 2 , Pedro M S D Cal 2 , Jean Bertoldo 1 , María Maneiro 3 , Elizabeth Perkins 4 , Julie Howard 5 , Michael J Deery 5 , Justin M Chalker 6 , Francisco Corzana 7 , Gonzalo Jiménez-Osés 7 , Gonçalo J L Bernardes 1, 2
Affiliation
|
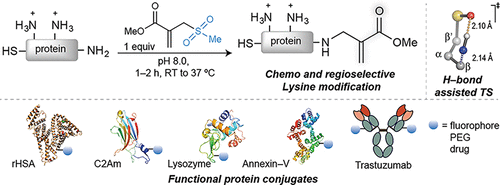
Site-selective chemical conjugation of synthetic molecules to proteins expands their functional and therapeutic capacity. Current protein modification methods, based on synthetic and biochemical technologies, can achieve site selectivity, but these techniques often require extensive sequence engineering or are restricted to the N- or C-terminus. Here we show the computer-assisted design of sulfonyl acrylate reagents for the modification of a single lysine residue on native protein sequences. This feature of the designed sulfonyl acrylates, together with the innate and subtle reactivity differences conferred by the unique local microenvironment surrounding each lysine, contribute to the observed regioselectivity of the reaction. Moreover, this site selectivity was predicted computationally, where the lysine with the lowest pKa was the kinetically favored residue at slightly basic pH. Chemoselectivity was also observed as the reagent reacted preferentially at lysine, even in those cases when other nucleophilic residues such as cysteine were present. The reaction is fast and proceeds using a single molar equivalent of the sulfonyl acrylate reagent under biocompatible conditions (37 °C, pH 8.0). This technology was demonstrated by the quantitative and irreversible modification of five different proteins including the clinically used therapeutic antibody Trastuzumab without prior sequence engineering. Importantly, their native secondary structure and functionality is retained after the modification. This regioselective lysine modification method allows for further bioconjugation through aza-Michael addition to the acrylate electrophile that is generated by spontaneous elimination of methanesulfinic acid upon lysine labeling. We showed that a protein–antibody conjugate bearing a site-specifically installed fluorophore at lysine could be used for selective imaging of apoptotic cells and detection of Her2+ cells, respectively. This simple, robust method does not require genetic engineering and may be generally used for accessing diverse, well-defined protein conjugates for basic biology and therapeutic studies.
中文翻译:

天然蛋白质的化学和区域选择性赖氨酸修饰
合成分子与蛋白质的位点选择性化学缀合扩展了它们的功能和治疗能力。当前基于合成和生化技术的蛋白质修饰方法可以实现位点选择性,但这些技术通常需要广泛的序列工程或仅限于 N 或 C 末端。在这里,我们展示了磺酰丙烯酸酯试剂的计算机辅助设计,用于修饰天然蛋白质序列上的单个赖氨酸残基。所设计的磺酰丙烯酸酯的这一特征,连同每个赖氨酸周围独特的局部微环境所赋予的先天和微妙的反应性差异,有助于观察到反应的区域选择性。此外,该位点选择性是通过计算预测的,其中 pKa 最低的赖氨酸是弱碱性 pH 下动力学上有利的残基。还观察到化学选择性,因为该试剂优先与赖氨酸反应,即使在存在其他亲核残基(如半胱氨酸)的情况下也是如此。在生物相容性条件(37°C,pH 8.0)下,反应速度快,并且使用单摩尔当量的磺酰丙烯酸酯试剂进行。该技术通过五种不同蛋白质的定量和不可逆修饰得到证明,包括临床使用的治疗性抗体曲妥珠单抗,无需事先进行序列工程。重要的是,它们的原生二级结构和功能在修改后得以保留。这种区域选择性赖氨酸修饰方法允许通过 aza-Michael 添加到丙烯酸酯亲电试剂中进行进一步的生物偶联,丙烯酸酯亲电试剂是在赖氨酸标记时通过自发消除甲亚磺酸产生的。我们表明,在赖氨酸处带有位点特异性荧光团的蛋白质-抗体偶联物可分别用于凋亡细胞的选择性成像和 Her2+ 细胞的检测。这种简单、稳健的方法不需要基因工程,通常可用于获取多种、明确定义的蛋白质偶联物,以进行基础生物学和治疗研究。我们表明,在赖氨酸处带有位点特异性荧光团的蛋白质-抗体偶联物可分别用于凋亡细胞的选择性成像和 Her2+ 细胞的检测。这种简单、稳健的方法不需要基因工程,通常可用于获取多种、明确定义的蛋白质偶联物,以进行基础生物学和治疗研究。我们表明,在赖氨酸处带有位点特异性荧光团的蛋白质-抗体偶联物可分别用于凋亡细胞的选择性成像和 Her2+ 细胞的检测。这种简单、稳健的方法不需要基因工程,通常可用于获取多种、明确定义的蛋白质偶联物,以进行基础生物学和治疗研究。
更新日期:2018-02-23
中文翻译:

天然蛋白质的化学和区域选择性赖氨酸修饰
合成分子与蛋白质的位点选择性化学缀合扩展了它们的功能和治疗能力。当前基于合成和生化技术的蛋白质修饰方法可以实现位点选择性,但这些技术通常需要广泛的序列工程或仅限于 N 或 C 末端。在这里,我们展示了磺酰丙烯酸酯试剂的计算机辅助设计,用于修饰天然蛋白质序列上的单个赖氨酸残基。所设计的磺酰丙烯酸酯的这一特征,连同每个赖氨酸周围独特的局部微环境所赋予的先天和微妙的反应性差异,有助于观察到反应的区域选择性。此外,该位点选择性是通过计算预测的,其中 pKa 最低的赖氨酸是弱碱性 pH 下动力学上有利的残基。还观察到化学选择性,因为该试剂优先与赖氨酸反应,即使在存在其他亲核残基(如半胱氨酸)的情况下也是如此。在生物相容性条件(37°C,pH 8.0)下,反应速度快,并且使用单摩尔当量的磺酰丙烯酸酯试剂进行。该技术通过五种不同蛋白质的定量和不可逆修饰得到证明,包括临床使用的治疗性抗体曲妥珠单抗,无需事先进行序列工程。重要的是,它们的原生二级结构和功能在修改后得以保留。这种区域选择性赖氨酸修饰方法允许通过 aza-Michael 添加到丙烯酸酯亲电试剂中进行进一步的生物偶联,丙烯酸酯亲电试剂是在赖氨酸标记时通过自发消除甲亚磺酸产生的。我们表明,在赖氨酸处带有位点特异性荧光团的蛋白质-抗体偶联物可分别用于凋亡细胞的选择性成像和 Her2+ 细胞的检测。这种简单、稳健的方法不需要基因工程,通常可用于获取多种、明确定义的蛋白质偶联物,以进行基础生物学和治疗研究。我们表明,在赖氨酸处带有位点特异性荧光团的蛋白质-抗体偶联物可分别用于凋亡细胞的选择性成像和 Her2+ 细胞的检测。这种简单、稳健的方法不需要基因工程,通常可用于获取多种、明确定义的蛋白质偶联物,以进行基础生物学和治疗研究。我们表明,在赖氨酸处带有位点特异性荧光团的蛋白质-抗体偶联物可分别用于凋亡细胞的选择性成像和 Her2+ 细胞的检测。这种简单、稳健的方法不需要基因工程,通常可用于获取多种、明确定义的蛋白质偶联物,以进行基础生物学和治疗研究。






























 京公网安备 11010802027423号
京公网安备 11010802027423号