当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the Decomposition Mechanisms of LiNH2, Mg(NH2)2, and NaNH2: A Joint Experimental and Theoretical Study
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-07-24 , DOI: 10.1021/acs.jpcc.9b03524 Huai-Jun Lin 1 , Peng Zhang 1 , Yan-Xiong Fang 2 , Yu-Jun Zhao 3 , Haichang Zhong 4 , Jia-Jun Tang 2, 3
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-07-24 , DOI: 10.1021/acs.jpcc.9b03524 Huai-Jun Lin 1 , Peng Zhang 1 , Yan-Xiong Fang 2 , Yu-Jun Zhao 3 , Haichang Zhong 4 , Jia-Jun Tang 2, 3
Affiliation
|
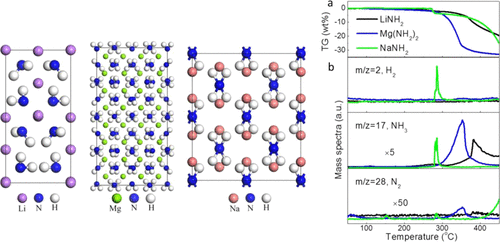
Metal amides are promising candidates for hydrogen storage, hydrogen production, NH3 synthesis and cracking, and so on. However, the decomposition behaviors and mechanisms of metal amides remain unclear. In this study, the decomposition properties of three metal amides, including LiNH2, Mg(NH2)2, and NaNH2, are studied by thermogravimetry, mass spectroscopy, and in situ X-ray diffraction techniques combined with density functional theory (DFT) calculations. It is found that Mg(NH2)2, LiNH2, and NaNH2 exhibit very different metal–N and N–H bond strengths, which precipitate various formations energies of different kinds of vacancies. As a result, LiNH2 releases a major amount of NH3, with a small amount of N2 at a temperature as high as 350 °C. Mg(NH2)2 releases NH3 and N2 synchronously at a temperature range of 300–400 °C without the emission of H2. NaNH2 synchronously releases H2, NH3, and a small amount of N2, at a narrow temperature range of 275–290 °C. Using DFT calculations, the decomposition behaviors and the corresponding decomposition mechanisms for LiNH2, Mg(NH2)2, and NaNH2 have been well understood.
中文翻译:

理解LiNH2,Mg(NH2)2和NaNH2的分解机理:联合实验和理论研究
金属酰胺是有望用于储氢,制氢,NH 3合成和裂解等的候选物。但是,金属酰胺的分解行为和机理尚不清楚。在这项研究中,通过热重分析,质谱分析和原位X射线衍射技术结合密度泛函理论(DFT)研究了LiNH 2,Mg(NH 2)2和NaNH 2三种金属酰胺的分解特性。)计算。发现Mg(NH 2)2,LiNH 2和NaNH 2表现出非常不同的金属-N和N-H键强度,这会沉淀出不同种类空位的各种形成能。结果,在高达350℃的温度下,LiNH 2释放大量的NH 3和少量的N 2。Mg(NH 2)2在300–400°C的温度范围内同步释放NH 3和N 2而不释放H 2。NaNH 2同步释放H 2,NH 3和少量N 2,在275–290°C的狭窄温度范围内。使用DFT计算,已经很好地了解了LiNH 2,Mg(NH 2)2和NaNH 2的分解行为和相应的分解机理。
更新日期:2019-07-25
中文翻译:

理解LiNH2,Mg(NH2)2和NaNH2的分解机理:联合实验和理论研究
金属酰胺是有望用于储氢,制氢,NH 3合成和裂解等的候选物。但是,金属酰胺的分解行为和机理尚不清楚。在这项研究中,通过热重分析,质谱分析和原位X射线衍射技术结合密度泛函理论(DFT)研究了LiNH 2,Mg(NH 2)2和NaNH 2三种金属酰胺的分解特性。)计算。发现Mg(NH 2)2,LiNH 2和NaNH 2表现出非常不同的金属-N和N-H键强度,这会沉淀出不同种类空位的各种形成能。结果,在高达350℃的温度下,LiNH 2释放大量的NH 3和少量的N 2。Mg(NH 2)2在300–400°C的温度范围内同步释放NH 3和N 2而不释放H 2。NaNH 2同步释放H 2,NH 3和少量N 2,在275–290°C的狭窄温度范围内。使用DFT计算,已经很好地了解了LiNH 2,Mg(NH 2)2和NaNH 2的分解行为和相应的分解机理。

































 京公网安备 11010802027423号
京公网安备 11010802027423号