当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Sultams and Cyclic N-Sulfonyl Ketimines via Iron-Catalyzed Intramolecular Aliphatic C–H Amidation
Organic Letters ( IF 4.9 ) Pub Date : 2019-07-12 00:00:00 , DOI: 10.1021/acs.orglett.9b01732
Dayou Zhong 1 , Di Wu 1 , Yan Zhang 1 , Zhiwu Lu 1 , Muhammad Usman 1 , Wei Liu 1 , Xiuqiang Lu 2 , Wen-Bo Liu 1, 3
Organic Letters ( IF 4.9 ) Pub Date : 2019-07-12 00:00:00 , DOI: 10.1021/acs.orglett.9b01732
Dayou Zhong 1 , Di Wu 1 , Yan Zhang 1 , Zhiwu Lu 1 , Muhammad Usman 1 , Wei Liu 1 , Xiuqiang Lu 2 , Wen-Bo Liu 1, 3
Affiliation
|
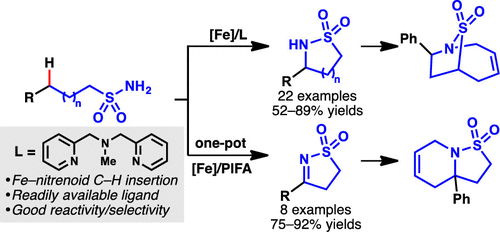
Cyclic sulfonamides (sultams) play a unique role in drug discovery and synthetic chemistry. A direct synthesis of sultams by an intramolecular C(sp3)–H amidation reaction using an iron complex in situ derived from Fe(ClO4)2 and aminopyridine ligand is reported. This strategy features a readily available catalyst and tolerates a broad variety of substrates as demonstrated by 22 examples (up to 89% yield). A one-pot iron-catalyzed amidation/oxidation procedure for the synthesis of cyclic N-sulfonyl ketimines is also realized with up to 92% yield (eight examples). The synthetic utility of the method is validated by a gram-scale reaction and derivatization of the products to ring-fused sultams.
中文翻译:

铁催化分子内脂肪族CH酰胺化合成Sultams和环状N-磺酰基酮亚胺
环磺酰胺(sultams)在药物发现和合成化学中起着独特的作用。据报道,使用Fe(ClO 4)2和氨基吡啶配体原位生成铁配合物,通过分子内C(sp 3)-H酰胺化反应可直接合成阿马丹。如22个实例所示,该策略的特点是易于获得的催化剂,并能耐受多种底物(产率高达89%)。还实现了用于合成环状N-磺酰基酮亚胺的一锅铁催化的酰胺化/氧化方法,产率高达92%(八个实例)。该方法的合成效用通过克级反应和将产物衍生为环稠合的杜仲而得到证实。
更新日期:2019-07-12
中文翻译:

铁催化分子内脂肪族CH酰胺化合成Sultams和环状N-磺酰基酮亚胺
环磺酰胺(sultams)在药物发现和合成化学中起着独特的作用。据报道,使用Fe(ClO 4)2和氨基吡啶配体原位生成铁配合物,通过分子内C(sp 3)-H酰胺化反应可直接合成阿马丹。如22个实例所示,该策略的特点是易于获得的催化剂,并能耐受多种底物(产率高达89%)。还实现了用于合成环状N-磺酰基酮亚胺的一锅铁催化的酰胺化/氧化方法,产率高达92%(八个实例)。该方法的合成效用通过克级反应和将产物衍生为环稠合的杜仲而得到证实。

































 京公网安备 11010802027423号
京公网安备 11010802027423号