当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efforts towards an On-Target Version of the Groebke-Blackburn-Bienaymé (GBB) Reaction for Discovery of Druglike Urokinase (uPA) Inhibitors.
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2019-08-29 , DOI: 10.1002/chem.201901917 Rafaela Gladysz 1 , Johannes Vrijdag 1, 2 , Dries Van Rompaey 1 , Anne-Marie Lambeir 2 , Koen Augustyns 1 , Hans De Winter 1 , Pieter Van der Veken 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2019-08-29 , DOI: 10.1002/chem.201901917 Rafaela Gladysz 1 , Johannes Vrijdag 1, 2 , Dries Van Rompaey 1 , Anne-Marie Lambeir 2 , Koen Augustyns 1 , Hans De Winter 1 , Pieter Van der Veken 1
Affiliation
|
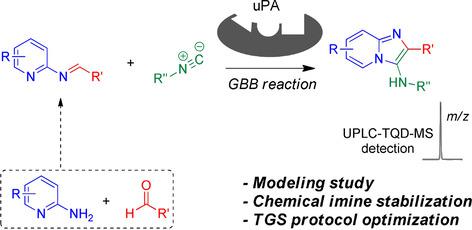
Target-guided synthesis (TGS) has emerged as a promising strategy in drug discovery. Although reported examples of TGS generally involve two-component reactions, there is a strong case for developing target-guided versions of three-component reactions (3CRs) because of their potential to deliver highly diversified druglike molecules. To this end, the Groebke-Blackburn-Bienaymé reaction was selected as a model 3CR. We recently reported a series of druglike urokinase inhibitors, and these serve as reference compounds in the present study. Due to the limited number of literature reports on target-guided 3CRs, multiple experimental parameters were optimized here. Most challenging was the formation of imine intermediates under near-physiological conditions. This aspect was addressed by exploring chemical imine stabilization strategies. Notably, imines are also crucial intermediates of other 3CRs. Such systematic studies are strongly required for further development of the TGS domain but are largely absent in the literature. Hence, this work is intended as a reference for future multicomponent-based TGS studies.
中文翻译:

努力开发针对目标的Groebke-Blackburn-Bienaymé(GBB)反应,以发现类似药物的尿激酶(uPA)抑制剂。
目标导向合成(TGS)已成为药物发现中一种有前途的策略。尽管已报道的TGS示例通常涉及两组分反应,但开发三靶标反应(3CR)的靶标指导方案的理由很强,因为它们具有传递高度多样化的类药物分子的潜力。为此,选择了Groebke-Blackburn-Bienaymé反应作为3CR模型。我们最近报道了一系列药物样尿激酶抑制剂,这些在本研究中用作参考化合物。由于有关目标导向3CR的文献报道数量有限,因此在此优化了多个实验参数。最具挑战性的是在接近生理条件下形成亚胺中间体。通过探索化学亚胺稳定策略解决了这一方面。尤其,亚胺也是其他3CR的关键中间体。TGS结构域的进一步发展强烈要求进行此类系统研究,但文献中基本上没有这种研究。因此,这项工作旨在为将来基于多成分的TGS研究提供参考。
更新日期:2019-08-29
中文翻译:

努力开发针对目标的Groebke-Blackburn-Bienaymé(GBB)反应,以发现类似药物的尿激酶(uPA)抑制剂。
目标导向合成(TGS)已成为药物发现中一种有前途的策略。尽管已报道的TGS示例通常涉及两组分反应,但开发三靶标反应(3CR)的靶标指导方案的理由很强,因为它们具有传递高度多样化的类药物分子的潜力。为此,选择了Groebke-Blackburn-Bienaymé反应作为3CR模型。我们最近报道了一系列药物样尿激酶抑制剂,这些在本研究中用作参考化合物。由于有关目标导向3CR的文献报道数量有限,因此在此优化了多个实验参数。最具挑战性的是在接近生理条件下形成亚胺中间体。通过探索化学亚胺稳定策略解决了这一方面。尤其,亚胺也是其他3CR的关键中间体。TGS结构域的进一步发展强烈要求进行此类系统研究,但文献中基本上没有这种研究。因此,这项工作旨在为将来基于多成分的TGS研究提供参考。































 京公网安备 11010802027423号
京公网安备 11010802027423号