当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Plasma-assisted catalytic formation of ammonia in N2–H2 plasma on a tungsten surface†
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2019-07-12 00:00:00 , DOI: 10.1039/c9cp01139k Marwa Ben Yaala 1, 2, 3, 4 , Arsalan Saeedi 1, 2, 3, 4 , Dan-Felix Scherrer 1, 2, 3, 4 , Lucas Moser 1, 2, 3, 4 , Roland Steiner 1, 2, 3, 4 , Marco Zutter 1, 2, 3, 4 , Martin Oberkofler 5, 6, 7 , Gregory De Temmerman 8, 9, 10, 11 , Laurent Marot 1, 2, 3, 4 , Ernst Meyer 1, 2, 3, 4
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2019-07-12 00:00:00 , DOI: 10.1039/c9cp01139k Marwa Ben Yaala 1, 2, 3, 4 , Arsalan Saeedi 1, 2, 3, 4 , Dan-Felix Scherrer 1, 2, 3, 4 , Lucas Moser 1, 2, 3, 4 , Roland Steiner 1, 2, 3, 4 , Marco Zutter 1, 2, 3, 4 , Martin Oberkofler 5, 6, 7 , Gregory De Temmerman 8, 9, 10, 11 , Laurent Marot 1, 2, 3, 4 , Ernst Meyer 1, 2, 3, 4
Affiliation
|
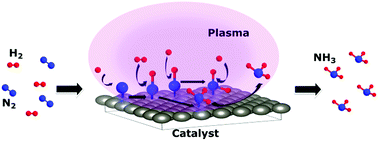
Plasma catalysis has drawn attention in the past few decades as a possible alternative to the Haber–Bosch process for ammonia production. In particular, radio frequency plasma assisted catalysis has the advantage of its adaptability to the industrial scale. However, in the past years, very few experimental studies have focused on the synthesis of ammonia from nitrogen/hydrogen radio frequency plasma. As a consequence, to date, there has been little agreement about the complex mechanisms underlying the radio frequency plasma-catalyst interactions. Gaining such an understanding is therefore essential for exploiting the potential of radio frequency plasma catalysis for ammonia production. In this study, we present results of ammonia formation from a nitrogen/hydrogen radio frequency plasma both without and with a tungsten catalyst for different initial nitrogen ratios. High yields of ammonia up to 32% at 25/75% of nitrogen/hydrogen were obtained using a combination of radio frequency low pressure plasma and a W surface as a catalyst. Furthermore, based on chemical analysis of the catalytic surface composition, a formation pathway of ammonia via the Eley–Rideal mechanism between adsorbed nitrogen and hydrogen from the gas phase is presented.
中文翻译:

钨表面上 N 2 -H 2等离子体中等离子体辅助催化形成氨†
在过去的几十年中,等离子体催化作为哈伯-博世(Haber-Bosch)制氨工艺的一种可能替代方法而受到关注。特别地,射频等离子体辅助催化具有其对工业规模的适应性的优点。然而,在过去的几年中,很少有实验研究集中于从氮/氢射频等离子体合成氨。结果,迄今为止,关于射频等离子体-催化剂相互作用的复杂机理几乎没有共识。因此,获得这种理解对于利用射频等离子体催化生产氨的潜力至关重要。在这项研究中,我们介绍了在不使用和使用钨催化剂的情况下,氮/氢射频等离子体针对不同的初始氮比形成氨的结果。使用射频低压等离子体和W表面作为催化剂,可以在25/75%的氮气/氢气下获得高达32%的氨高产率。此外,基于催化表面成分的化学分析,氨的形成途径通过Eley-Rideal机理介绍了气相中吸附的氮和氢之间的关系。
更新日期:2019-07-12
中文翻译:

钨表面上 N 2 -H 2等离子体中等离子体辅助催化形成氨†
在过去的几十年中,等离子体催化作为哈伯-博世(Haber-Bosch)制氨工艺的一种可能替代方法而受到关注。特别地,射频等离子体辅助催化具有其对工业规模的适应性的优点。然而,在过去的几年中,很少有实验研究集中于从氮/氢射频等离子体合成氨。结果,迄今为止,关于射频等离子体-催化剂相互作用的复杂机理几乎没有共识。因此,获得这种理解对于利用射频等离子体催化生产氨的潜力至关重要。在这项研究中,我们介绍了在不使用和使用钨催化剂的情况下,氮/氢射频等离子体针对不同的初始氮比形成氨的结果。使用射频低压等离子体和W表面作为催化剂,可以在25/75%的氮气/氢气下获得高达32%的氨高产率。此外,基于催化表面成分的化学分析,氨的形成途径通过Eley-Rideal机理介绍了气相中吸附的氮和氢之间的关系。

































 京公网安备 11010802027423号
京公网安备 11010802027423号